Abstract
Recent studies in our laboratory have demonstrated a role of nitric oxide (NO) in morphine-induced reduction of intraocular pressure (IOP) and pupil diameter (PD) in the New Zealand white (NZW) rabbit. The present study was designed to determine the effect of morphine on NO release in the aqueous humor of NZW rabbits, as this effect could be associated with morphine-mediated changes in aqueous humor dynamics and iris function. Dark adapted NZW rabbits were treated as follows: 1) treatment with morphine (10, 33 or 100 μg, 5 min); 2) treatment with morphine or endomorphin-1 for 5, 15 or 30 min; 3) pretreatment with naloxone (100 μg), L-NAME (125 μg) or reduced glutathione (GSH, 100 μg) for 30 minutes, followed by treatment with morphine (100 μg, 5 min). After the various treatment regimens, aqueous humor samples were obtained by paracenthesis and immediately assayed for nitrates and nitrites (an index of NO production), using a microplate assay kit. Morphine caused a dose-dependent increase in the levels of NO in aqueous humor after 5 min of treatment with each dose. Rabbits treated with endomorphin-1 (100 μg) had no significant change in NO levels in aqueous at any point in the time course. Aqueous samples from rabbits treated with morphine (100 μg) for 5 minutes increased from 29.84 ± 2.39 μM (control) to 183.94 ± 23.48 μM (treated). The increase in NO levels by morphine (100 μg, 5 min) was completely inhibited in the presence of naloxone (100 μg), L-NAME (125 μg) or GSH (100 μg). These results indicate that morphine-induced increase in NO production in aqueous humor is a transient response that is linked to activation of mu opioid receptors. Data obtained suggest that morphine-stimulated changes in ocular hydrodynamics and iris function are due, in part, to increased release of NO in aqueous humor. In addition, the sensitivity of the response to L-NAME and GSH suggests that morphine-induced release of nitric oxide into aqueous humor is mediated by activation of mu-3 opioid receptors found in the anterior segment of the eye.
Keywords: morphine, nitric oxide, aqueous humor, mu-3 receptors, opioids
1. Introduction
Morphine is a prototypical mu opioid receptor agonist that is used clinically as an analgesic agent. At present, the mu opioid receptor has three recognized subtypes, mu-1, mu-2, and the most recently ascribed mu-3 receptor. The mu-3 subtype is an opiate alkaloid selective receptor that is insensitive to opiate peptides like endomorphin-1 and -2. Correlated with its insensitivity to opiate peptides, the mu-3 receptor is also coupled to constitutive NO synthase (cNOS) derived NO release (Stefano et al., 1993). Morphine-induced NO release is naloxone sensitive and is antagonized by the NOS inhibitors N-nitro-L-arginine and Nω-nitro-L-arginine methyl ester (L-NAME). Furthermore, the mu-3 receptor appears to be more sensitive to inactivation by reduced glutathione (GSH) than are classical mu, delta and kappa opioid receptors.
Although morphine-induced reduction of intraocular pressure (IOP) and pupil diameter (PD) has been previously reported, the exact mechanism(s) involved in these actions has not been completely elucidated. Initial studies by Myashita (1913) demonstrated that morphine raised the IOP, but subsequent reports revealed morphine’s ability to lower IOP (Leopold and Comroe, 1948; Faniciullacci et al., 1980; Drago et al., 1985; Dortch-Carnes and Russell, 2006; Bonfiglio et al., 2006). The ocular effects of morphine (particularly the miosis), are generally thought to occur primarily through centrally-mediated signaling mechanisms (Lee and Wang, 1975; Murray, et al., 1983). To date, however, a direct intraocular component has not been ruled out.
Aqueous humor is produced by the ciliary epithelial cells located in the ciliary body (Krupin et al., 1986). IOP is determined primarily by the dynamic equilibrium between the production of aqueous humor in the ciliary body and its efflux mainly through the trabecular meshwork and Schlemm’s canal (Wiederholt et al., 1991). Because recent studies in our laboratory (Dortch-Carnes and Russell, 2006) and others (Bonfiglio et al., 2006) have generated evidence of a role of NO in morphine-mediated reduction of IOP and PD, the present study is designed to determine the effect of morphine on NO formation in aqueous humor, because this effect could play a role in morphine-mediated reduction of IOP and PD. With the use of an inhibitor of NO synthesis and the sulfhydryl agent, GSH, additional evidence that morphine-mediated changes in NO levels in aqueous humor are linked to activation of mu-3 opioid receptors has also been established.
2. Materials and Methods
2.1. Animals
Male, dark-adapted (reverse light cycle, 12 h dark/12 h light) New Zealand white (NZW) rabbits, weighing approximately 2 - 3 kg, were used in this study. Drug applications and collection of aqueous humor samples were done in the dark under a constant red light, as was done when measuring IOP in previous studies (Dortch-Carnes and Russell, 2006) because dark adapted rabbits have higher IOP values (Rowland et al 1981; Bar-Ilan, 1984; Rowland et al., 1986; Smith and Gregory, 1989). All animals were housed in a controlled environment individually at room temperature and were maintained on Purina rabbit chow and tap water ad libitum. Animal care and treatment were in accordance with the ARVO (Association for Research in Vision and Ophthalmology) Resolution on the Use of Animals in Research, and the Institutional Care and Use Committee of the Morehouse School of Medicine, Atlanta, GA., USA, reviewed and approved protocols. Endomorphin-1 was obtained from Tocris (Ellisville, MO., USA) and all other drugs were from Sigma-Aldrich (St. Louis, MO., USA). All drugs were dissolved in distilled H2O.
2.2. Drug Administration
Morphine (10, 33, 50 and 100 μg/25 μl vehicle) and endomorphin-1 (100 μg/25 μl vehicle) were applied topically and bilaterally into the eyes of individual rabbits. Aqueous humor was removed at 5, 15 or 30 minute time points after administration of the respective drug. In other experiments, the nonselective opioid receptor antagonist naloxone (100 μg), the nonselective NOS inhibitor L-NAME (125 μg), or the sulfhydryl reagent, GSH (100 μg) were instilled topically into each eye 0.5 h before morphine (100 μg) administration. The doses of antagonists used were chosen based on other studies (Drago et al., 1980; Giuffrida et al., 2003) and previous studies in our laboratory (Dortch-Carnes and Russell, 2006) that demonstrated effective inhibition of morphine and NO-mediated signaling at the selected doses. At the end of drug treatments, rabbits were euthanized by i.v. administration of 0.5 ml beuthanasia-D containing pentobarbital sodium (390 mg/ml) and phenytoin sodium (50 mg/ml).
2.3. Measurement of NO levels
NO is a very unstable molecule in solution with a half-life of only a few seconds. Therefore, in these studies NO was measured as its stable metabolites, nitrate and nitrite. In the cell, NO undergoes a series of reactions with several molecules present in biological fluids and is eventually metabolized to nitrite (NO2−) and nitrate (NO3−). Immediately following sacrifice, aqueous humor samples were obtained by paracenthesis and transferred to microfuge tubes containing a protease inhibitor mix. All aqueous samples were kept on ice and immediately assayed for NO (nitrates + nitrites) levels using a microplate assay from Active Motif, Carlsbad, California. The principle of the NO quantitation kit is that nitrate in the sample is converted to nitrite in the presence of nitrate reductase and cofactors. Then, nitrate and nitrite levels are assayed using Griess Reagent.
2.4. Statistics
Data were analyzed for differences using one-way analysis of variance followed by the Holm-Sidak method for multiple comparisons. Results are expressed as mean values ± SEM obtained from 8 control or 4 – 7 drug-treated rabbits and were considered significant when P < 0.05.
3. Results
Morphine (100 μg) applied topically and bilaterally caused a dose-dependent (Fig. 1) and time-sensitive (Fig. 2A) increase in aqueous humor NO levels. Aqueous humor samples taken after 5 min of morphine treatment showed a significant increase in the levels of NO compared to control animals (Fig. 2A). After 15 or 30 min of treatment, however, levels of NO were diminished back to basal levels (Fig. 2A). In contrast to morphine (an opiate alkaloid), endomorphin-1 (an opiate peptide), did not cause an increase in NO levels in aqueous samples at any of the time points tested (Fig. 2B).
Fig. 1.
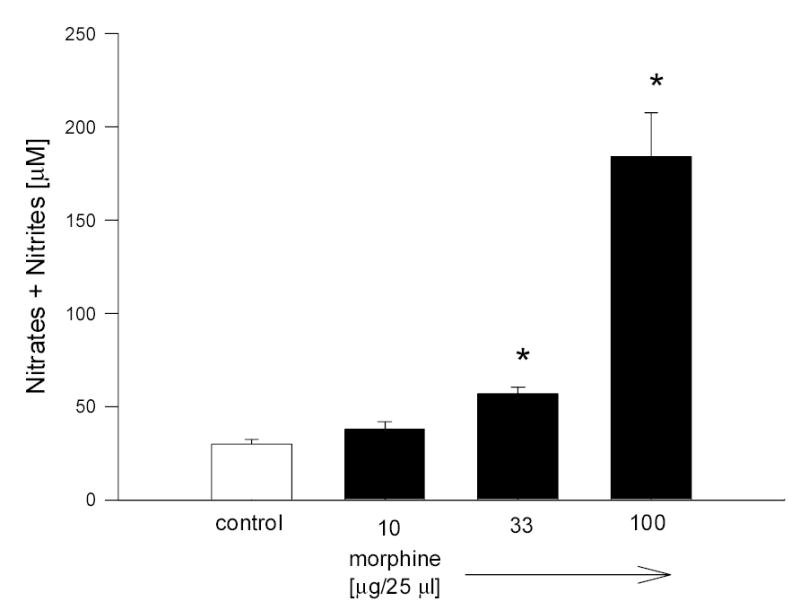
Dose response of morphine on NO production in rabbit aqueous humor. Data are mean ± SEM of 8 (control) or 4 (drug treated) animals. * P < 0.05 compared to control.
Fig. 2.
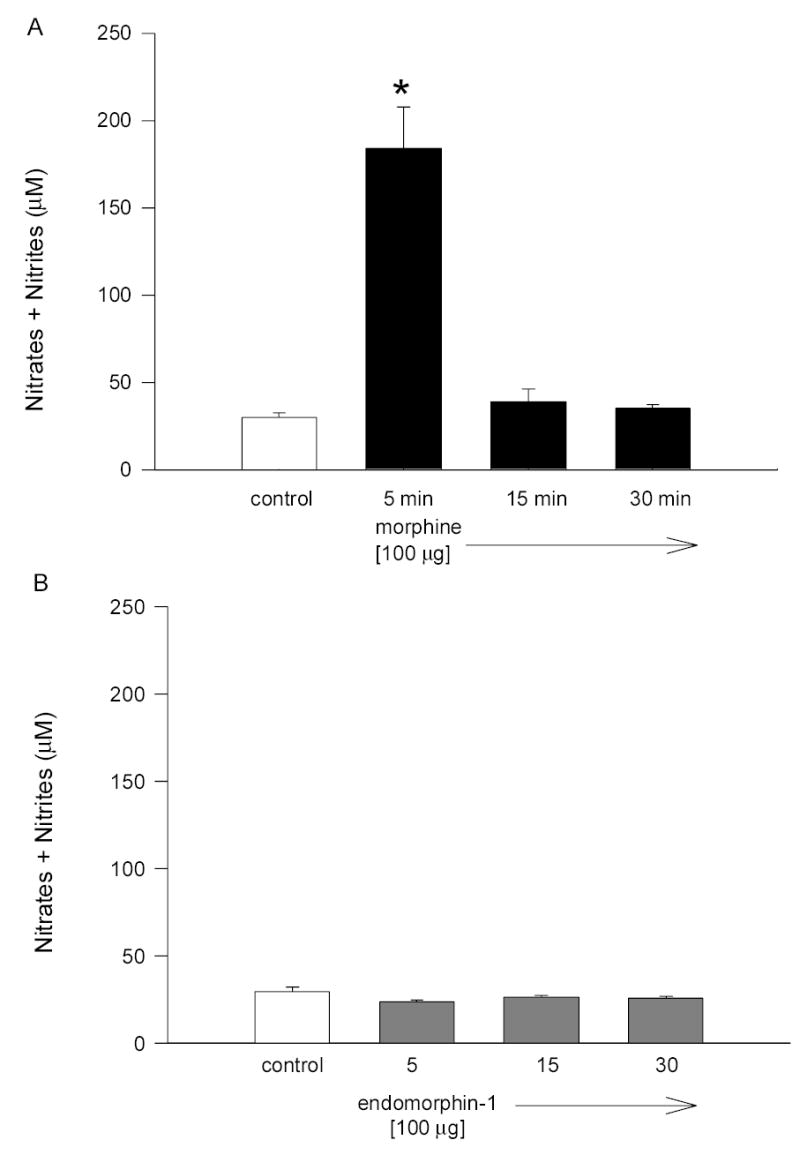
Time course of the effect of morphine (A) and endomorphin-1 (B) on NO levels in rabbit aqueous humor. Data are mean ± SEM of 8 (control) or 4 –7 (drug treated) animals. *P < 0.05 compared to control.
In order to confirm that morphine-stimulated NO release in aqueous was mediated by activation of opioid receptors, the opioid receptor antagonist, naloxone was utilized. Naloxone alone did not significantly alter NO levels in aqueous as compared to control. However, pretreatment of animals with naloxone (100 μg, 30 min), resulted in complete inhibition of morphine-induced increases in NO levels in aqueous (Fig. 3).
Fig. 3.
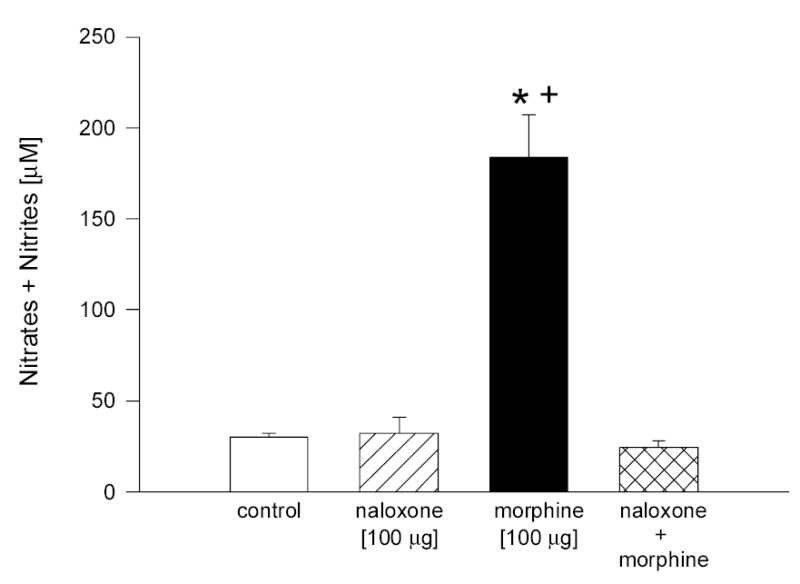
Effect of naloxone on morphine-induced increase in NO levels in rabbit aqueous humor. Animals were pretreated with naloxone for 30 min, followed by treatment with morphine for 5 min. Data are mean ± SEM of 8 (control) or 4 – 6 (drug treated) animals. *P < 0.05, compared to control; +P < 0.05, compared to naloxone + morphine.
To provide evidence that the morphine-induced increase in the levels of NO in aqueous humor is associated with activation of receptors linked to NO release, experiments using the nonselective NOS inhibitor, L-NAME, and the sulfhydryl compound, GSH, were performed. Although both GSH and L-NAME were administered topically to the rabbit eyes in absence of a stabilizer, no irritation to the animals was seen. When administered alone, neither agent caused a significant change in NO levels present in aqueous. Pretreatment with either compound, however, resulted in complete inhibition of morphine-stimulated NO release into aqueous humor (Fig. 4, L-NAME; Fig. 5, GSH).
Fig. 4.
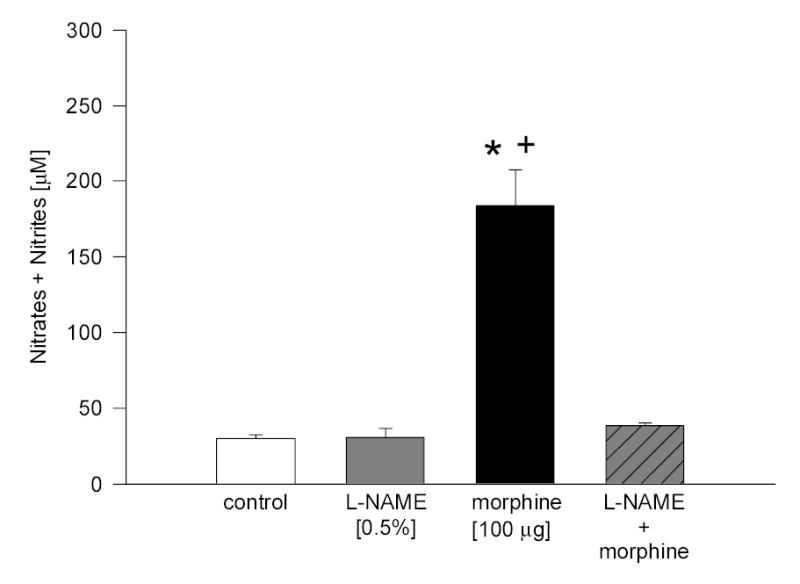
Effect of L-NAME on morphine-induced increase in NO level in rabbit aqueous humor. Animals were pretreated with L-NAME for 30 min, followed by treatment with morphine for 5 min. Data are mean ± SEM of 8 (control) or 4 – 5 (drug treated) animals. * P < 0.05, compared to control; + P < 0.05, compared to L-NAME + morphine.
Fig. 5.
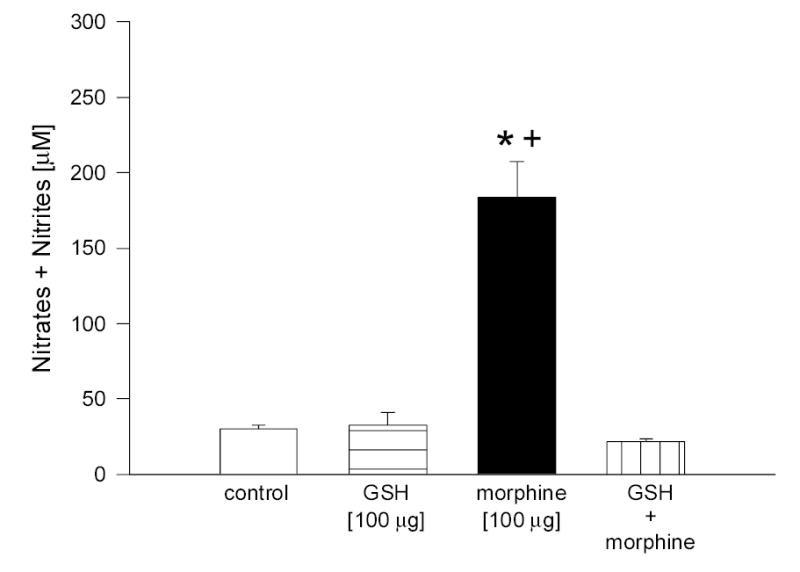
Effect of GSH on morphine-induced increase in NO levels in rabbit aqueous humor. Animals were pretreated with GSH for 30 min, followed by treatment with morphine for 5 min. Data are mean ± SEM of 8 (control) or 4 – 5 (drug treated) animals. * P< 0.05, compared to control; + P < 0.05, compared to GSH + morphine.
4. Discussion
Morphine is known to mediate its effects through activation of mu opioid receptors (Matthes et al., 1996). Of the three currently recognized mu opioid receptor subtypes (mu-1, mu-2 and mu-3), the mu-3 subtype is the only one which is known to be opiate alkaloid selective and coupled to NO synthase derived NO release (Stefano, 1999). In addition, the mu-3 receptor appears to be much more sensitive to inactivation by reduced glutathione (GSH) than other mu, delta or kappa opioid receptors (Makman et al., 1996).
Because morphine’s effects on other biological systems have been consistently linked to NO release and signaling (Stefano et al., 1996; Prevot et al., 1998; Stefano, 1999), and since our previous studies demonstrate a role of NO in morphine-induced reduction of intraocular pressure (IOP) and pupil diameter (PD), this study was designed to determine the effect of morphine on the levels of NO in aqueous humor, as this effect could be associated with morphine-mediated changes in aqueous humor dynamics and iris function.
In the present study, morphine caused a dose-dependent but transient increase in the level of NO in aqueous humor. Tseng et al. (2000) found that both dopamine and morphine stimulated NO production in glandular epithelial cells transiently, with peak effect occurring within 30 seconds. In addition, NO levels returned to basal by approximately 40 seconds.
The opiate peptide, endomorphin-1, was used in this study to demonstrate inactivity of opiate peptides at mu-3 receptors because it is commonly used by other investigators (Stefano, 1999; Rialas et al., 2000; Stefano et al., 2004) for that purpose. As expected, the peptide did not stimulate release of NO into aqueous humor at any of the time points used, thereby indicating inactivity at mu-3 receptors. This finding is also in agreement with other studies that have demonstrated the inability of opiate peptides to stimulate NO release in various biological systems (Stefano et al., 1995; Cadet et al., 2003; Stefano et al., 2004).
Morphine (100 μg) increased the levels of NO from 29.84 ± 2.39 μM (control) to 183.94 ± 23.48 μM (treated). Some investigators have found similar basal levels of NO in human aqueous humor samples (Kotikoski et al., 2002). In a separate study, Kotikiski and collegues (2002) demonstrated close to three-fold higher basal levels of NO (assayed as nitrates and nitrites) in rabbit aqueous humor as compared to the basal levels obtained in the present study. Furthermore, Kotikoski and collegues showed that the levels of NO increased from 80.9 ± 38.8 μM (untreated) to 179.4 μM (drug treated) 2 hr after topical administration of sodium nitroprusside. The observed variations in basal levels of NO found in the aqueous humor of the NZW rabbit may be due to differences in sensitivity of the NO detection methods used.
Preliminary studies in our laboratory have provided evidence of a role of NO in the ocular hypotensive and miotic effects of morphine (Dortch-Carnes and Russell, 2006). In addition, our previous studies have demonstrated that morphine caused bilateral reductions in IOP and PD that were similar in magnitude, suggesting that the action may involve activity on mu receptors located centrally and peripherally. Indeed, morphine exerts a reduction in pupil diameter that is principally dependent on central mechanisms (e.g. action on the mesencephalon, Edinger-Westphal nucleus), but at least in part mediated by opioid receptors located in the iris muscle that have been recognized as being of the mu type (Drago et al., 1980; Fanciullacci et al., 1980a; Murray et al., 1983). The same holds true for the IOP lowering effect of morphine (Fanciullacci et al., 1980b; Drago et al., 1985). Besides, various opioid peptides have been reported in the iris-ciliary body of different species (Fanciullacci et al., 1980b; Drago et al., 1984a, b; 1985). Thus opioid receptors of the mu type possibly located in the coroidal tissue (iris-ciliary body) are involved in the ocular effects of morphine. Because both eyes were drug treated in the present study, no conclusions can be drawn regarding whether or not the morphine-induced NO release in aqueous humor seen in this study is a central or peripheral mediated response. The present study, however, has provided additional evidence of morphine’s ability to release NO, possibly from the ciliary epithelial cells, and other sites, into the aqueous humor.
Morphine produced maximal reductions in IOP and PD after 30 minutes of treatment. Since no measurements of either of those parameters in the presence of morphine were taken prior to the 30 min time-point in those experiments, no direct correlation of these events to morphine-stimulated NO release in aqueous humor can be made. However, it can be said that morphine-induced NO release does occur prior to the maximal reduction in the IOP and PD.
Although the effects seen with morphine and endomorphin-1 were consistent with other studies involving real-time measurements of NO release (Cadet et al., 2003; Stefano et al., 1995; Tseng et al., 2000), it is also possible that the effect of morphine on NO levels demonstrated in this study could be due to the dark adaptation of the animals. The rabbits used in the present study were dark adapted because it has been consistently demonstrated that rabbits have higher IOP values in the dark than in the light (Rowland et al 1981; Bar-Ilan, 1984; Rowland et al., 1986; Smith and Gregory, 1989). Because this method of artificially raising the baseline IOP values in the previous study was used, it was also done in the present study for consistency.
Although the IOP values were artificially raised, the dark adapted animals are not representative models of ocular hypertension (experimental glaucoma). Studies that have reported on the role of NO in the regulation of aqueous humor outflow and IOP have generated conflicting data. Researchers who have conducted studies using rabbits with experimental glaucoma have shown that topical application of a nitric oxide synthase inhibitor produced a reduction in IOP (Giuffrida et al., 2003). Others have reported that topical NO donors increased the IOP without affecting aqueous humor formation (Larsson et al., 1995) or outflow facility (Krupin et al., 1977). Still, other studies have shown that NO donors have no effect on the IOP in rabbits (Taniguchi et al., 1998) or humans (Kiss et al., 1999).
After establishing the optimal incubation time (5 min), required to get maximal stimulation of NO production in aqueous humor in the presence of morphine (100 μg), experiments examining the effect of naloxone (nonselective opioid receptor antagonist), L-NAME (nonselective NO-synthase inhibitor), or GSH (sulfhydryl agent) on morphine-induced production of NO were performed. Morphine-stimulated NO release was completely inhibited in the presence of naloxone (100 μg), L-NAME (125 μg) and GSH (100 μg). When administered alone, none of the agents produced any significant change in the levels of NO in aqueous.
In summary, morphine caused an increase in the levels of NO found in aqueous humor that was opiate alkaloid specific as well as naloxone and GSH sensitive. This morphine-induced release of NO into the aqueous humor is, therefore, likely to be mediated by activation of mu-3 opiate receptors. The proposed signaling events thought to be involved in morphine-induced reduction of IOP and PD start with morphine binding to mu-3 opioid receptors in the anterior segment of the eye. Activation of mu-3 receptors results in release of NO, subsequent increase in cGMP formation, followed by reduction of IOP and PD. The inability of the opiate peptide, endomorphin-1 to stimulate NO release into aqueous as well as the sensitivity of the response to GSH further solidifies the role of mu-3 receptors in morphine-induced reduction of IOP and PD. Future studies are aimed at determining the site(s) of NO production as well as the location of the mu-3 opioid receptors involved in morphine-induced ocular hypotension and miosis.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Eye Institute (5R03 EY14346) and the technical assistance of Tisha Moore Johnson.
Footnotes
The author has no financial or proprietary interest in any product mentioned herin.
Supported by an NIH grant from the National Eye Institute (5R03EY14346)
References
- Bar-Ilan A. Diurnal and seasonal variations in intraocular pressure in the rabbit. Exp Eye Res 1984. 1984;39:175–181. doi: 10.1016/0014-4835(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Bonfiglio V, Bucolo C, Camillieri G, Drago F. Possible involvement of nitric oxide in morphine-induced miosis and reduction of intraocular pressure in rabbits. Eur J Pharmacol. 2006;534 (1–3):227–232. doi: 10.1016/j.ejphar.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Cadet P, Mantione KJ, Stefano GB. Molecular identification and functional expression of μ3, a novel alternatively spliced variant of the human μ opiate receptor gene. J Immunol. 2003;170:5118–5123. doi: 10.4049/jimmunol.170.10.5118. [DOI] [PubMed] [Google Scholar]
- Dortch-Carnes J, Russell KRM. Morphine-induced reduction of intraocular pressure and pupil diameter: role of nitric oxide. Pharmacol. 2006;77:17–24. doi: 10.1159/000091993. [DOI] [PubMed] [Google Scholar]
- Drago F, Gorgone G, Spina F, Panissidi G, Dal Bello A, Moro F, Scapaginini U. Opiate receptors in the rabbit iris. Naunyn-Schmiedeberg’s Arch Pharmacol. 1980;315:1–4. doi: 10.1007/BF00504223. [DOI] [PubMed] [Google Scholar]
- Drago F, Facchinetti F, Petraglia F, Panissidi GB, Bellomia F, Genazzani AR, Scapagnini R. Ocular opioids: distribution and function. In: Muller EE, Genzzani AR, editors. Central and Peripheral Endorphins: Basic and Clinical Aspects. Raven Press; New York: 1984a. pp. 151–155. [Google Scholar]
- Drago F, Gorgone G, Dal Bello A, Cavaliere S, Facchinetti F, Genazzani AR, Scapagnini R. Endorphins in the eye: evidence for a possible local action. In: Fraioli F, Isidori A, editors. Opioid Peptides in Periphery. Raven Press; New York: 1984b. pp. 119–126. [Google Scholar]
- Drago F, Panissidi G, Bellomio F, Dal Bello A, Aguglia E, Gorgone G. Effects of opiates and opioids on intraocular pressure of rabbits and humans. Clin Exp Pharmacol Physiol. 1985;12:107–13. doi: 10.1111/j.1440-1681.1985.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Fanciulacci M, Boccuni M, Pietrini U, Sicuteri F. Search for opiate receptors in human pupil. Int J Clin Pharmacol Res. 1980a;1:109–113. [Google Scholar]
- Fanciulacci M, Boccuni M, Pietrini U, Sicuteri F. The naloxone conjunctival test in morphine addiction. Eur J Pharmacol. 1980b;61:319–20. doi: 10.1016/0014-2999(80)90136-3. [DOI] [PubMed] [Google Scholar]
- Giuffrida F, Bucolo C, Drago F. Topical application of a nitric oxide synthase inhibitor reduces intraocular pressure in rabbits with experimental glaucoma. J Ocul Pharmacol. 2003;19 (6):527–534. doi: 10.1089/108076803322660440. [DOI] [PubMed] [Google Scholar]
- Kiss B, Dallinger S, Findl O, Rainer G, Eichler HG, Schmetterer L. Acetazolamide-induced cerebral and ocular vasodilation in humans is independent of nitric oxide. Am J Physiol. 1999;276:R1661–R1667. doi: 10.1152/ajpregu.1999.276.6.R1661. [DOI] [PubMed] [Google Scholar]
- Kotikoski H, Moilanen E, Vapaatalo H, Aine E. Biochemical markers of the L-arginine-nitric oxide pathway in the aqueous humour in glaucoma patients. Acta Ophthalmol Scand. 2002;80:191–195. doi: 10.1034/j.1600-0420.2002.800214.x. [DOI] [PubMed] [Google Scholar]
- Kotikoski H, Alajuuma P, Moilanen E, Salmenpera P, Oksala O, Laippala Pl, Vapaatalo H. Comparison of nitric oxide donors in lowering intraocular pressure in rabbits: role of cyclic GMP. J Ocul Pharmacol Therap. 2002;18(1):11–23. doi: 10.1089/108076802317233171. [DOI] [PubMed] [Google Scholar]
- Krupin T, Weiss A, Becker B, Holmberg N, Fritz C. Increased intraocular pressure following topical azide or nitroprusside. Invest Ophthalmol Vis Sci. 1977;16:1002–1007. [PubMed] [Google Scholar]
- Krupin T, Wax M, Moolchandani J. Aqueous production. Trans Ophthalmol Soc UK. 1986;105:111–116. [PubMed] [Google Scholar]
- Larsson LI, Maus T, Brubaker RF, Nathanson JA. Topically applied hydralazine: effects on systemic cardiovascular parameters, blood-aqueous barrier, and aqueous humor dynamics in normotensive humans. J Ocul Pharmacol Therapeut. 1995;11:145–156. doi: 10.1089/jop.1995.11.145. [DOI] [PubMed] [Google Scholar]
- Lee HK, Wang SC. Mechanism of morphine-induced miosis in the dog. J Pharmacol Exp Ther. 1975;192:415–31. [PubMed] [Google Scholar]
- Leopold IH, Comroe JH. Effect of intramuscular administration of morphine, atropine, scopolamine and neostigmine on the human eye. Arch Ophthalmol. 1948;40:285–91. doi: 10.1001/archopht.1948.00900030291006. [DOI] [PubMed] [Google Scholar]
- Makman MH, Dobrenis K, Downie S, Lyman WD, Dvorkin B. Presence of opiate alkaloid-selective μ3 in cultured astrocytes and in brain and retina. Adv Exp Biol and Med. 1996;402:23–8. doi: 10.1007/978-1-4613-0407-4_4. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, LeMeur M, Dolle P, Tzavara E, Honoune J, roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383 (6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Murray RB, Adler MW, Korczyn AD. Minireview. The pupillary effects of opioids. Life Sci. 1983;33:495–509. doi: 10.1016/0024-3205(83)90123-6. [DOI] [PubMed] [Google Scholar]
- Myashita H. Ocular tension. Zentralblatt fur Biochemie und Biophysik. 1913;15:95–8. [Google Scholar]
- Prevot V, Rialas CM, Croix D, Salzet M, Dupouy JP, Poulain P, Beauvillain JC, Stefano GB. Morphine and anandamide coupling to nitric oxide stimulates GnRH and CRF release from rat median eminence: neurovascular regulation. Brain Res. 1998;790 (1–2):236–244. doi: 10.1016/s0006-8993(98)00066-3. [DOI] [PubMed] [Google Scholar]
- Rialas CM, Weeks B, Cadet P, Goumon Y, Stefano GB. Nociceptin, endomorphin-1 and -2 do not interact with invertebrate immune and neural mu 3 opiate receptor. Acta Pharmacol Sin. 2000;21(6):516–520. [PubMed] [Google Scholar]
- Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- Rowland JM, Sawyer WK, Tittel J, Ford CJ. Studies on the circadian rhythm of IOP in rabbits: correlation with aqueous inflow and cAMP content. CurrEye Res. 1986;5:201–206. doi: 10.3109/02713688609020044. [DOI] [PubMed] [Google Scholar]
- Smith SD, Gregory DA. Circadian rhythm of aqueous flow underlies the circadian rhythm of IOP in NZW rabbits. Invest Ophthalmol Vis Sci. 1989;30:775–778. [PubMed] [Google Scholar]
- Stefano GB, Digenis A, Spector S, Leung MK, Bilfinger TV, Makman MH, Charrer B, Abumrad NN. Opiate-like substances in an invertebrate, an opiate receptor on invertebrate and human immunocytes, and a role in immunosuppression. Proc Natl Acad Sci U S A. 1993;90 (23):11099–103. doi: 10.1073/pnas.90.23.11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Hartman A, Bilfinger TV, Magazine HI, Lius Y, Casares F, Goligorsky MS. Presence of the μ3 opiate receptor in endothelial cells. J Biol Chem. 1995;270:30290–30293. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Scharrer B, Smith EM, Hughes TK, Jr, Magazine HI, Bilfinger TV, Hartman AR, Fricchione GL, Liu Y, Makman MH. Opioid and opiate immunoregulatory processes. Crit Rev Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- Stefano GB. The μ3 opiate receptor subtype. Pain Forum. 1999;8:206–209. [Google Scholar]
- Stefano GB, Zhu W, Cadet P, Bilfinger TV, Mantione K. Morphine enhances nitric oxide release in the mammalian gastrointestinal tract via the μ3 opiate receptor subtype: a hormonal role for endogenous morphine. J Physiol Pharmacol. 2004;55:279–288. [PubMed] [Google Scholar]
- Taniguchi T, Kawakami H, Sawada A, Iwaki M, Tsuji A, Sugiyama K, Kitazawa Y. Effects of nitric oxide synthase inhibitor on intraocular pressure and ocular inflammation following laser irradiation in rabbits. Curr Eye Res. 1998;17:308–315. doi: 10.1076/ceyr.17.3.308.5225. [DOI] [PubMed] [Google Scholar]
- Tseng L, Mazella J, Goligorsky MS, Rialas CM, Stefano GB. Dopamine and morphine stimulate nitric oxide release in human endometrial glandular epithelial cells. J Soc Gynecol Investig. 2000;7:343–347. [PubMed] [Google Scholar]
- Wiederholt M, Helbig H, Korbmacher C. Ion transport across the ciliary epithelium: lessons from cultured cells and proposed role of the carbonic anhydrase. In: Botre F, Gross G, Story BT, editors. Carbonic Anhydrase. VCH; Weinheim: 1991. pp. 232–244. [Google Scholar]


