The “oxyradical hypothesis” of myocardial stunning proposes that superoxide, released on reperfusion, leads to contractile dysfunction via the production of the more reactive hydroxyl free radical from the iron catalysed Haber-Weiss reaction. However, superoxide reacts many times faster with nitric oxide (NO), than with ferric iron, leading to the formation of peroxynitrite (ONOO−), which is a potent oxidising and nitrating agent with properties and reactivity similar to hydroxyl radicals. Consequently ONOO− is a candidate for the initiation of oxidation reactions following brief ischaemia–reperfusion injury. The production of reactive nitrogen intermediates such as ONOO− is reflected in vivo by the formation of the amino acid derivative 3-nitrotyrosine. Recently, we have developed a sensitive method for the quantitative analysis of tissue nitrotyrosine which avoids the artefactual formation of nitrotyrosine that can occur with more conventional protein preparative steps.1 The present study sought to test the hypothesis that myocardial stunning causes increased formation of tissue nitrotyrosine.
METHODS
Large White pigs (mean (SD) weight 38 (4) kg) were anaesthetised and prepared as previously described2 to allow temporary occlusion of the circumflex coronary artery with simultaneous recording of haemodynamic parameters, circumflex coronary blood flow (group A only) and regional and distal myocardial contractile function by sonomicrometric segment shortening. Following 30 minutes of stabilisation, regional ischaemia–reperfusion was induced by 10 cycles of two minutes vessel occlusion and two minutes reperfusion. Subsequently animals were monitored for 30 minutes (group A) (n = 10) or six hours (group B) (n = 7).
Tissue nitrotyrosine and tyrosine concentrations were measured blindly in myocardial samples from stunned and non-ischaemic control areas (determined ex vivo by the distribution of the circumflex and anterior descending coronary arteries, respectively) from all animals. Measurements were carried out using stable isotope dilution gas chromatography mass spectrometry following extraction of tissue proteins and alkaline hydrolysis.1 The latter is preferable to acid hydrolysis, since it avoids artefactual formation of nitrotyrosine by acid and nitrite/nitrate.
Comparison of tissue [nitrotyrosine] in stunned and non-ischaemic myocardium was made using Wilcoxon's signed rank test. Comparison of changes in segment shortening were tested using paired t tests for within group differences and unpaired t tests for between group differences. Significance was accepted at p < 0.05.
RESULTS
The effects of stunning on systemic haemodynamics and regional segment shortening up to six hours have been reported previously.2 Briefly, measurements of segment shortening demonstrated a significant fall in function in the ischaemic territory compared with baseline (mean (SEM) 57 (5)% of baseline function, p < 0.0001) and with non-ischaemic myocardium (63 (6)% of non-ischaemic function, p < 0.001) 30 minutes after the stunning stimulus when coronary flow had returned to normal (26 (3) v 32 (3) ml/min, p = ns). Function in group B animals recovered to 92 (7)% of baseline 5.5 hours later. During coronary occlusion, segment shortening in the ischaemic territory became negative, indicating systolic bulging of the ventricular wall. Non-ischaemic segment function showed a small, non-significant, compensatory increase in function during vessel occlusion.
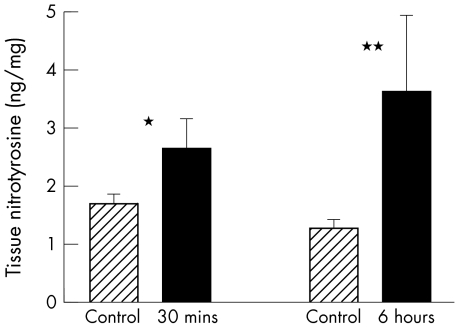
Stunned myocardium was found to have a significantly greater concentration of nitrated tyrosine compared with internal controls both after 30 minutes (2.7 (0.5) v 1.7 (0.1) ng/mg dry weight of protein, p < 0.05) and six hours (3.6 (1.3) v 1.3 (0.1) ng/mg, p < 0.02) of reperfusion (fig 1).
Figure 1.
Nitrotyrosine formation in stunned myocardium. Myocardial concentrations of nitrotyrosine were significantly higher in stunned compared with remote non-ischaemic tissue at 30 minutes and six hours post-reperfusion. *p < 0.05 versus non-ischaemic internal tissue; **p < 0.02 versus non-ischaemic internal control tissue.
DISCUSSION
This study shows that repetitive episodes of ischaemia–reperfusion, culminating in myocardial stunning, cause an increased formation of nitrotyrosine in cardiac tissue. Nitrotyrosine can be formed in a variety of ways, by reaction of ONOO− with tyrosine residues, by reaction of nitrite (the major end product of NO metabolism) with hypochlorous acid to form reactive nitrogen species or reaction of nitrite with proteins under acidic conditions. The latter two pathways, however, are unlikely to have occurred in this study. Firstly, the hypochlorous dependent reaction is reliant on myeloperoxidase or eosinophil peroxidase activity and histological examination of our samples (data not shown) did not reveal any inflammatory cell infiltrate. Secondly, the acidic pathway requires an extremely low pH (< 2.5), which does not occur in viable myocardium. The increase in tissue nitrotyrosine is therefore consistent with the production of reactive nitrogen species such as ONOO− from the reaction of superoxide anion and NO and is further indirect evidence for the role of reactive oxygen species in the pathophysiology of myocardial stunning.
Although this is the first time nitrotyrosine has been quantified chemically in cardiac tissue, other studies support this finding and indicate a possible role for ONOO− in the development of the contractile dysfunction of stunning. In particular, immunocytochemical evidence of tyrosine nitration has been reported following worsening of myocardial stunning in response to l-arginine3 and impairment of cardiac contractile efficiency in response to exogenous ONOO− formation seen in isolated rat hearts.4 It is also of interest that desferrioxamine and n-2-mercaptopropionylglycine, which have previously been shown to reduce stunning,5 are potent inhibitors of ONOO− initiated oxidation.6,7
Potential mechanisms for ONOO− induced contractile dysfunction include the nitration and oxidation of both lipids and proteins. Targets shown to be specifically vulnerable to such damage include actin, sarcoplasmic reticulum Ca2+ adenosinetriphosphatase and α tubulin, a cellular protein that undergoes an enzymatically regulated tyrosination/detyrosination cycle.
Although this study supports a role for ONOO− in the genesis of stunning, recovery of function in our model was not dependent upon the clearance of nitrated tyrosine residues from the myocardium. This finding would suggest that ONOO− may be causing myocardial damage by attacking cellular targets other than tyrosine residues, as outlined above, and that nitrotyrosine is solely a footprint of ONOO− formation. Alternatively, de novo protein synthesis could lead to contractile recovery without a requirement for the immediate removal of proteins damaged by tyrosine nitration.
Despite the assumption that ONOO− production is detrimental to cellular function, there is evidence that this may not always be the case. For example, following myocardial infarction ONOO− has been proposed to have a protective effect. However, although such an action following stunning cannot be excluded by the present data, protection in the context of infarction appears to be mediated via the inhibition of neutrophils, a cell type known not to be involved in the pathogenesis of stunning.
Abbreviations
NO, nitric oxide
ONOO−, peroxynitrite
Sources of support: British Heart Foundation Junior Fellowship (FS/97006).
REFERENCES
- 1.Frost MT, Halliwell B, Moore KP. Analysis of free and protein-bound nitrotyrosine in human plasma by a gas chromatography/mass spectrometry method that avoids nitration artifacts. Biochem J 2000;345(pt 3):453–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Baker CSR, Rimoldi O, Camici PG, et al. Repetitive myocardial stunning in pigs is associated with the increased expression of inducible and constitutive nitric oxide synthases. Cardiovasc Res 1999;43:685–97. [DOI] [PubMed] [Google Scholar]
- 3.Mori E, Haramaki N, Ikeda H, et al. Intra-coronary administration of L-arginine aggravates myocardial stunning through the production of peroxynitrite in dogs. Cardiovasc Res 1998;40:113–23. [DOI] [PubMed] [Google Scholar]
- 4.Schulz R, Dodge KL, Lopaschuk GD, et al. Peroxynitrite impairs cardiac contractile function by decreasing cardiac efficiency. Am J Physiol 1997;272:H1212–19. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. Mechanisms of myocardial stunning. Circulation 1990;82:723–38. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990;87:1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radi R, Beckman JS, Bush KM, et al. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 1991;266:4244–50. [PubMed] [Google Scholar]