Abstract
Objective: To test the hypothesis that, when measured in the long axis, left ventricular systolic function is abnormal in patients with diastolic heart failure.
Design: A case–control study.
Setting: University teaching hospital (tertiary referral centre).
Patients: 68 patients with heart failure, 29 with a left ventricular ejection fraction (LVEF) of > 0.45 and diastolic dysfunction (diastolic heart failure), 39 with an LVEF of ≤ 0.45 (systolic heart failure), and 105 normal subjects, including 33 age matched controls.
Methods: LVEF was measured by cross sectional Simpson's method, and mitral annular amplitudes and velocities by M mode and tissue Doppler echocardiography, respectively, along with mitral Doppler inflow velocities. Results were compared between the three groups.
Main outcome measures: Peak systolic mitral annular velocity and amplitude between the different groups.
Results: The mitral annular peak mean velocity and amplitude in systole were lower in the patients with diastolic heart failure (mean (SEM), 4.8 (0.2) cm/s) than in the age matched normal controls (6.1 (0.14) cm/s), but higher than those with systolic heart failure (2.8 (0.13) cm/s) (all p < 0.001). Similar changes were seen the mitral annular amplitude during systole. Peak early diastolic velocity and amplitude were also significantly reduced in the group with diastolic heart failure. Left ventricular hypertrophy was evident in over 95% patients in both diastolic and systolic heart failure groups, with a comparable left ventricular mass index.
Conclusions: In patients with diastolic heart failure and evidence of left ventricular hypertrophy, there is systolic left ventricular impairment as measured by myocardial Doppler imaging of the longitudinal axis. Thus subtle abnormalities of systolic function are present in patients with heart failure and a normal left ventricular ejection fraction, and there appears to be a continuum of systolic function between those with truly normal, mildly impaired (labelled diastolic heart failure), and obviously abnormal left ventricular systolic function. Isolated diastolic dysfunction is uncommon.
Keywords: diastole, systole, heart failure, long axis
Heart failure with a normal left ventricular ejection fraction is usually called “diastolic heart failure” if there are no other obvious causes. In some parts of the world, so called diastolic heart failure may be more common than systolic heart failure in patients presenting with heart failure symptoms.1 The distinction between the two states is based upon the measurement of left ventricular ejection fraction (LVEF), usually by echocardiography. In patients with a normal or nearly normal LVEF it is assumed that as there is “preserved systolic function” the primary disorder is diastolic. Although LVEF assesses global function, it is a relatively crude measure of left ventricular systolic function. Measurement of the ventricular long axis velocities and amplitude using tissue Doppler and M mode imaging of the mitral annulus is thought to provide a more sensitive index of systolic function than LVEF.2 Using these techniques we sought to determine whether ventricular systolic function was truly normal in patients with “diastolic heart failure” or presumed isolated diastolic dysfunction.
METHODS
Study subjects
We studied 101 subjects. Of these, 29 (mean age 70.4 years, range 37–82 years) presented with heart failure and an LVEF of > 0.45 (diastolic heart failure), 39 (mean age 65.4 years, range 36–87 years) presented with systolic heart failure (LVEF < 0.45), and there were 33 age matched normal controls (mean age 64.9 years, range 49–84 years). The latter were selected from among 105 normal subjects from the local community (mean age 47.3 years, range 20–84 years) in view of the important impact of age on diastolic and systolic function.3 The diagnostic criteria for diastolic heart failure used were those published by the European study group on diastolic heart failure.4
Normal subjects had no cardiovascular symptoms, no history of hypertension, diabetes mellitus, hypercholesterolaemia, or peripheral vascular disease, and a normal ECG and cross sectional echocardiogram.
Echocardiography
Echocardiograms were obtained using a GE-VingMed system 5 echocardiographic machine (GE-VingMed Sound AB, Horten, Norway) with a 3.5 mHz multiphase array probe in subjects lying in the left lateral decubitus position. The echocardiographic techniques and calculations of different cardiac dimensions were performed according to the recommendations of the American Society of Echocardiography.5–8 The ejection fraction was obtained using a modified biplane Simpson's method from apical two and four chamber views (LV2D) and also by using the atrioventricular plane displacement method described by Willenheimer and colleagues.9 Left ventricular hypertrophy was defined as a left ventricular mass index of ≥ 131 g/m2 in men and ≥ 100 g/m2 in women, in accordance with Framingham criteria.10 All echocardiographic studies were done when patients were clinically stable on treatment.
Recording of myocardial tissue velocities
Myocardial velocities were recorded by pulsed wave Doppler. The meridional mitral annular velocities were recorded from the apical window with the Doppler sample volume placed in the septal and lateral aspects of the mitral annulus in the four chamber view, and anterobasal and inferior aspects in the two chamber view. All images were acquired during end expiration, and attention was paid to obtaining the appropriate velocity range settings (high enough pulse repetition frequency) to avoid aliasing. Three cardiac cycles of M mode tracing and myocardial velocity were recorded and transferred to the off line workstation with specific computer software for further analysis.
Recording of mitral annular excursion was by M mode from the same four positions in the four chamber and two chamber apical views.
Measurements
Measurements were made off line on three consecutive beats on M mode images and two consecutive beats of myocardial Doppler studies, and the average of the two to three beats was used for the analysis. The onsets of each of the waveforms were obtained by extrapolating the initial portion of the acceleration slope to the baseline. In a similar manner, the endings of the waveforms were obtained by extrapolating the terminal portion of the deceleration slope to the baseline.
From each long axis M mode trace, we measured the mitral annular excursion in systole (SLAX), early diastolic excursion (ELAX), and late diastolic annular motion (ALAX) as a result of atrial contraction in anterior, septal, lateral, and posterior left ventricular longitudinal axes. The deceleration time (DTLAX) of the early mitral annular diastolic excursion (ELAX) was measured from the onset of lengthening to the onset of diastasis on the recordings. Similarly, the peak of the systolic apically directed (Sm wave), early diastolic (Em), and late diastolic (Am) myocardial velocities and the isovolumic relaxation time (IVRTm) were recorded in the same left ventricular long axes as above. Early (EMV) and late (AMV) transmitral flow velocities, the ratio of early to late peak velocities (E/AMV), the deceleration time of EMV velocity (DTMV), and the isovolumic relaxation time (IVRTMV) were also obtained from the cross sectional pulsed Doppler flow across the mitral valve and the left ventricular outflow tract.
Statistical analysis
Differences in various dependent variables of the echocardiographic indices between the two heart failure groups and the normal age matched control group were determined by analysis of variance for continuous variables (ANOVA). Multiple comparisons as post hoc tests were performed if ANOVA probability values were highly significant, to ascertain the significance of the mean difference between different paired groups of normal controls, patients with diastolic heart failure, or patients with systolic heart failure. Interobserver and intraobserver reliability was assessed in 30 randomly chosen patients using inter- and intraclass correlations and Bland–Altman methods.11 The acquired images were analysed independently by two blinded observers to obtain interobserver reliability. The results are expressed as mean (SEM). Differences are considered significant at p < 0.05.
RESULTS
Table 1 shows the main or most obvious causes of heart failure in the three groups. A history of myocardial infarction was more commoner in systolic heart failure than in diastolic heart failure (p = 0.04). Hypertension was common in both groups and overall was the most common risk factor for heart failure in all patients. Left ventricular hypertrophy was evident in over 95% of the patients in both the diastolic and the systolic heart failure groups, with a comparable left ventricular mass index (diastolic heart failure, 261.4 (35.3) g/m2; systolic heart failure, 249.1 (12.4) g/m2; p = 0.66).
Table 1.
Causes of heart failure in the study groups
| Cause of failure | DHF | SHF | Total |
| Coronary artery disease | 9/29 (31%) | 17/39 (44%) | 26/68 (38%) |
| History of old MI | 2/29 (7%) | 13/39 (33%) | 15/68 (22%) |
| Hypertension | 15/29 (52%) | 16/39 (41%) | 31/68 (48%) |
| Diabetes mellitus | 8/29 (28%) | 18/39 (46%) | 26/68 (38%) |
| LVH | 28/29 (96%) | 37/39 (95%) | 65/68 (96%) |
DHF, diastolic heart failure; LVH, left ventricular hypertrophy, defined as left ventricular mass index ≥131 g/m2 in men and ≥100 g/m2 in women; MI, myocardial infarction; SHF, systolic heart failure.
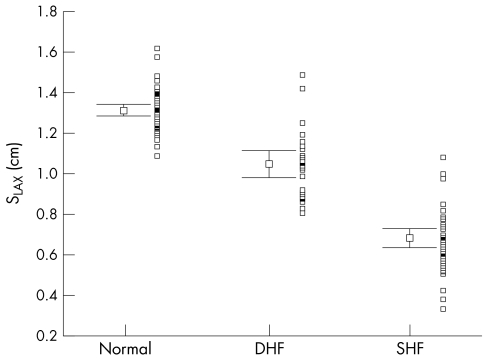
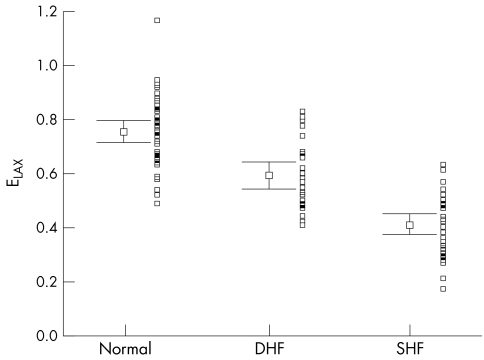
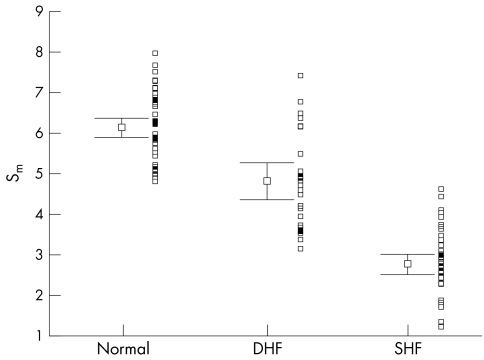
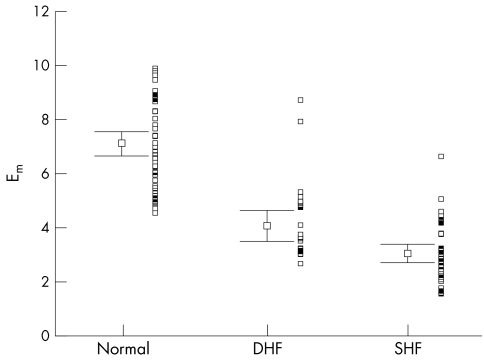
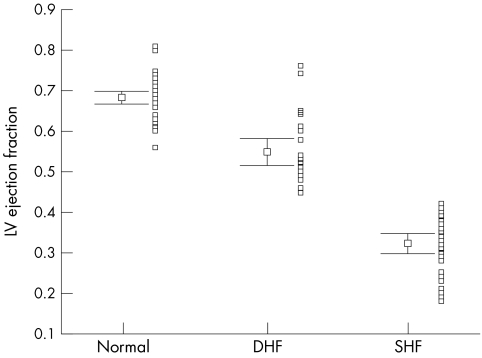
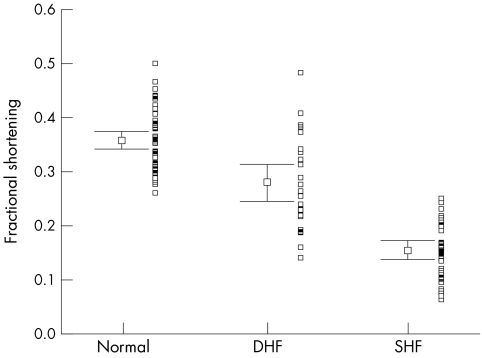
Table 2 summarises the main echocardiographic and Doppler tissue imaging variables in all subjects, including the entire control population of 105 normal subjects and the 33 age matched controls. There were no differences in age (p = 0.112) and heart rate (p = 0.57) between the three groups. However, the left ventricular ejection fraction, mitral annular systolic (SLAX) and diastolic excursions (E/ALAX) and velocities (Sm, Em, Am), and deceleration and isovolumic relaxation times (DT, IVRT) in these three groups all showed significant differences (all p < 0.001). The values from the diastolic heart failure group were generally intermediate between the normal and the systolic heart failure groups, except Em in the diastolic heart failure group, which had a value closer to the systolic heart failure group. The individual values are shown in figs 1–6. When using the mean ± 2 SD of the age matched control group as a normal range, 38% of the diastolic heart failure group had Sm values below the normal range, while 45% had a subnormal Em value, assuming a lower limit of normal for Sm and Em of 4.5 and 3.6 cm/s, respectively.
Table 2.
Doppler echocardiographic results in the three groups
| Age matched normal controls (n=33) | Range (2 SD) in 105 normal subjects | DHF | SHF | p Value (ANOVA) | |
| Age (years) | 64.9 (1.46) | 18.1–76.2 | 70.4 (2.07) | 65.4 (2.09) | >0.112 |
| Heart rate (beats/min) | 73.2 (2.17) | 51.4–92.0 | 77.3 (3.12) | 78.5 (1.88) | <0.053 |
| LVEDD (cm) | 4.52 (0.07) | 3.54–5.55 | 5.16 (0.24) | 6.27 (0.13) | <0.001 |
| LVEF (2D) | 0.67 (0.01) | 0.56–0.82 | 0.54 (0.02) | 0.32 (0.01) | <0.001 |
| LVEFAVPD | 0.66 (0.01) | 0.55–0.86 | 0.53 (0.02) | 0.33 (0.01) | <0.001 |
| FS | 0.35 (0.01) | 0.24–0.50 | 0.28 (0.02) | 0.15 (0.01) | <0.001 |
| E/AMV | 0.97 (0.05) | 0.6–1.76 | 0.92 (0.13) | 1.66 (0.14) | <0.001 |
| DTMV (s) | 0.19 (0.01) | 0.11–0.25 | 0.26 (0.02) | 0.18 (0.01) | <0.001 |
| IVRTMV (s) | 0.09 (0.01) | 0.07–0.11 | 0.14 (0.03) | 0.10 (0.01) | >0.027 |
| SLAX (cm) | 1.30 (0.02) | 1.05–1.74 | 1.05 (0.03) | 0.69 (0.03) | <0.001 |
| ELAX (cm) | 0.74 (0.03) | 0.49–1.33 | 0.59 (0.03) | 0.41 (0.02) | <0.001 |
| ALAX (cm) | 0.55 (0.01) | 0.37–0.69 | 0.52 (0.02) | 0.32 (0.02) | <0.001 |
| DTLAX (s) | 0.12 (0.00) | 0.09–0.15 | 0.16 (0.01) | 0.15 (0.01) | <0.001 |
| Sm (cm/s) | 6.10 (0.14) | 4.73–9.24 | 4.81 (0.23) | 2.76 (0.13) | <0.001 |
| Em (cm/s) | 6.78 (0.28) | 4.57–12.83 | 4.11 (0.29) | 3.10 (0.19) | <0.001 |
| Am (cm/s) | 7.91 (0.25) | 4.57–10.27 | 7.15 (0.33) | 3.91 (0.25) | <0.001 |
| IVRTm (s) | 0.72 (0.03) | 0.38–0.96 | 1.03 (0.02) | 1.09 (0.05) | <0.009 |
Values are mean (SEM).
ALAX, late diastolic annular motion; Am, late diastolic myocardial velocity by colour Doppler myocardial imaging; ANOVA, analysis of variance; DHF, diastolic heart failure; DTLAX, deceleration time of early mitral annular diastolic excursion by long axis M mode echocardiography; DTMV, deceleration time of peak early Doppler mitral filling velocity; E/AMV, ratio of early to late Doppler mitral inflow velocities; ELAX, early mitral diastolic excursion by long axis M mode echocardiography; Em, early diastolic myocardial velocity by colour Doppler myocardial imaging; FS, fractional shortening; IVRTm, isovolumic myocardial relaxation time by colour Doppler myocardial imaging; IVRTMV, isovolumic relaxation time from the Doppler mitral and aortic flow pattern; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; LVEFAVPD, left ventricular ejection fraction from atrioventricular plane method; SHF, systolic heart failure; SLAX, mitral annular excursion in systole by long axis M mode echocardiography; Sm, mitral annular velocities by colour Doppler myocardial imaging; 2D, modified cross sectional Simpson's method.
Figure 1.
Distribution of systolic mitral annular amplitudes by long axis M mode echocardiography (SLAX) with mean (SEM) and adjacent scatterplot in all three groups of patients. DHF, patients with diastolic heart failure; SHF, patients with systolic heart failure.
Figure 2.
Distribution of early diastolic mitral annular amplitudes by long axis M mode echocardiography (ELAX) with mean (SEM) and adjacent scatterplot in all three groups of patients.
Figure 3.
Distribution of peak systolic mitral annular amplitudes by colour Doppler myocardial imaging (Sm) with mean (SEM) and adjacent scatterplot in all three groups of patients.
Figure 4.
Distribution of peak early diastolic mitral annular amplitudes by colour Doppler myocardial imaging (Em) with mean (SEM) and adjacent scatterplot in all three groups of patients.
Figure 5.
Distribution of left ventricular (LV) ejection fraction by modified cross sectional Simpson's method with mean (SEM) and adjacent scatterplot in all three groups of patients.
Figure 6.
Distribution of fractional shortening with mean (SEM) and adjacent scatterplot in all three groups of patients.
Table 3 shows the various drug treatments used in each heart failure group. None is likely to have affected the results; in particular the use of β blockers and calcium antagonists was low in the diastolic heart failure group.
Table 3.
Drug treatments used in the study groups
| DHF | SHF | Total | |
| Diuretics | 19/29 (66%) | 35/39 (90%) | 54/68 (79%) |
| β Blockers | 3/29 (10%) | 15/39 (38%) | 18/68 (26%) |
| Calcium channel antagonists | 4/29 (14%) | 6/39 (15%) | 10/68 (15%) |
| ACEI/AIIRA | 14/29 (48%) | 31/39 (79%) | 45/68 (66%) |
| Nitrates | 18/29 (62%) | 33/39 (85%) | 51/68 (75%) |
| Digoxin | 3/29 (10%) | 5/39 (13%) | 8/68 (12%) |
| Spironolactone | 6/29 (21%) | 6/39 (15%) | 12/68 (18%) |
| Amiodarone | 1/29 (3%) | 4/39 (10%) | 5/68 (7%) |
| Statins | 4/29 (14%) | 7/39 (18%) | 11/68 (16%) |
ACEI, angiotensin converting enzyme inhibitor; AIIRA, angiotensin II receptor antagonist; DHF, diastolic heart failure; SHF, systolic heart failure.
The intraclass correlations for various mitral annular variables by the same observer were between 0.8 and 0.9. The interobserver correlations for same variables were between 0.7 and 0.9. With the Bland–Altman method, the mean difference between observations was less than 5% of the mean value of the observations for measurements of amplitude, duration, and velocity.
DISCUSSION
In this study, left ventricular systolic function in patients with “diastolic heart failure” was reduced in comparison with age matched normal controls when measured in the long axis by tissue Doppler imaging or M mode, and in about 40% of cases it was below the normal range for all age groups. Patients with diastolic heart failure had better systolic function than patients with systolic heart failure. Their systolic function appeared to be intermediate between control subjects with truly normal systolic function and the patients with systolic heart failure and obvious severe impairment of function. The reduction in systolic mitral annular amplitude and velocity in the long axis was not compensated for by circumferential shortening, as evidenced by a similar reduction in fractional shortening in all the heart failure subjects. The left ventricular long axis thus appears to be a particularly sensitive measure of ventricular systolic function, reflecting the subendocardial position of the longitudinal fibres which makes them more vulnerable to ischaemia, left ventricular hypertrophy, and other abnormalities of activation and relaxation.2
None of the drugs used by the patients with diastolic heart failure was likely to have affected left ventricular systolic function; in particular the use of β blockers was less in the diastolic failure group than in the systolic failure group, and the use of calcium channel blockers was infrequent and similar in both types of heart failure (14% and 15% in diastolic and systolic failure, respectively).
Thus in many patients with so called diastolic heart failure there is impairment of systolic function when this is assessed by measuring changes in the left ventricular long axis using tissue Doppler or M mode echocardiography, although by definition all these patients had a “normal” left ventricular ejection fraction (> 0.45). Although the LVEF is a measure of global left ventricular function and may be a useful screening test, it is generally a poor and non-specific index of systolic function. Furthermore, the definition of diastolic heart failure according to the European study group on diastolic heart failure4—namely, normal or mildly reduced left ventricular systolic function (for example, a left ventricular ejection fraction of ≥ 45%)—already implies the possible coexistence of mildly impaired systolic function and a diastolic abnormality, even though such patients are considered to have “primary diastolic heart failure.”
Our findings differ from those of Gandhi and colleagues,12 who recently reported a study on a group of patients with acute hypertensive pulmonary oedema and found that left ventricular ejection fraction was normal on admission before treatment. They concluded that the left sided heart failure was caused by exacerbation of diastolic dysfunction by hypertension and not by transient systolic dysfunction or mitral regurgitation. However, if they had assessed the long axis movement by tissue Doppler imaging, the more subtle reduction in the left ventricular longitudinal function in systole, as well as in early diastole, could have been detected and may be equally relevant to the development of pulmonary oedema.13
Our results are compatible with what is known about the underlying physiology of ventricular relaxation. There is a substantial body of experimental evidence that ventricular relaxation should be considered as part of ventricular systole.14 Conceptually it is very difficult to separate relaxation from contraction, and it is better to consider them together as part of a continuous cycle. The energy generated during systole is stored within the myocardium, particularly in coiled collagen fibres, and following myocyte relaxation the ventricle uncoils, creating left ventricular suction. As myocyte contractile function declines, recoil will also decline in parallel. This decline may be exacerbated by independent changes in the extracellular matrix.15 In comparison with the controls, the early diastolic velocity (Em) was reduced to a greater extent than Sm in the diastolic heart failure group in our study, indicating that factors other than intrinsic recoil or stored energy from the previous systole influence the left ventricular long axis velocity in early diastole.
Our results have an impact on the concept of diastolic heart failure. There has been much debate about this condition and even suggestions that many patients with this diagnostic label may not have left ventricular failure at all.16,17 Obesity may compound the difficulties of assessment.18 Moreover, basing the distinction between systolic and diastolic heart failure on the ejection fraction is rather arbitrary. The problem of the diagnosis of diastolic heart failure is confounded by the difficulty in proving non-invasively that left ventricular diastolic dysfunction is present—over and above what would be expected in normal aging or from the effects of hypertension and left ventricular hypertrophy—and is the cause of the patient's symptoms.17 There may also be differences in the aetiology. It appears that “diastolic heart failure” is probably the early stage of hypertensive heart failure and the associated left ventricular hypertrophy, with both diastolic and systolic components, whereas the more severe forms of systolic heart failure are more commonly caused by large myocardial infarcts with remodelling. It remains to be shown whether the treatments that are effective in systolic heart failure will also be effective in diastolic heart failure.
Limitations
We tried to achieve a case–control study by matching age and heart rate, as diastolic function declines with aging, and preload is dependent on heart rate. However, afterload was not necessarily matched in all three groups. Furthermore, neither left ventricular end diastolic internal dimension index (LVEDIDI < 3.2 cm/m2 ) nor left ventricular end diastolic volume index (LVEDVI < 102 ml/m2) were matched between the normal and the diastolic heart failure groups in order to exclude the possibility of diastolic left ventricular dysfunction secondary to a high end systolic load and volume. However, subjects suffering from significant valvar heart disease were excluded from the study. In the systolic heart failure group there was a lower prevalence of coronary artery disease and myocardial infarction than might typically be found in a Western population, and left ventricular hypertrophy was present in most of the patients, which probably reflects a greater impact of hypertension in this population as a cause of heart failure.19 Neither of these factors is likely to affect the results as long axis function is known to be depressed in patients with ischaemic heart failure.2
The range of the LVEF and LVEDD given as mean and SEM in the aged matched normal controls is small and there is no overlap with the diastolic heart failure group. However, scatter plots (figs 1–6) showing the range of values for M mode left ventricular dimensions and cross sectional left ventricular systolic functional indices do show some overlap of these variables between the normal and diastolic heart failure groups, and also between the diastolic and systolic heart failure groups.
Although the number of patients in this study was small, the results were highly significant, with most of the comparisons achieving a statistical power of more than 80%. Additional numbers of subjects are unlikely to affect the results significantly.
Conclusions
In patients with diastolic heart failure and evidence of left ventricular hypertrophy, there is some degree of systolic left ventricular impairment. This can be detected by measuring the left ventricular long axis by tissue Doppler echocardiography of mitral annular velocity, and mitral annular amplitude by M mode. Diastolic heart failure appears to be part of a continuum between normal and severely impaired left ventricular systolic function. Isolated diastolic heart failure is less common than previously thought.
Acknowledgments
We are grateful to Mr K K Wong MPhil, from Centre for Clinical Trials and Epidemiological Research, Chinese University of Hong Kong, for his statistical advice and helpful assistance.
Abbreviations
ALAX, late diastolic annular motion by long axis M mode echocardiography
Am, late diastolic myocardial velocity by colour Doppler myocardial imaging
AMV, late transmitral flow velocity
DT, deceleration time in early filling phase
DTLAX, deceleration time of early mitral annular diastolic excursion
DTMV, deceleration time of the peak early Doppler mitral filling velocity
E/AMV, ratio of early to late Doppler mitral inflow velocities
ELAX, early mitral diastolic excursion by long axis M mode echocardiography
Em, early diastolic myocardial velocity by colour Doppler myocardial imaging
EMV, early transmitral flow velocity
FS, fractional shortening
IVRTm, isovolumic myocardial relaxation time by colour Doppler myocardial imaging
IVRTMV, isovolumic relaxation time from the Doppler mitral and aortic flow pattern
LVEFAVPD, left ventricular ejection fraction from atrioventricular plane method
LVEDD, left ventricular end diastolic dimension
LVEDVI, left ventricular end diastolic volume index
LVEF, left ventricular ejection fraction
LVEDIDI, left ventricular end diastolic internal dimension index
SLAX, mitral annular excursion in systole by long axis M mode echocardiography
Sm, mitral annular velocities by colour Doppler myocardial imaging
REFERENCES
- 1.Yip GWK, Ho PPY, Woo KS, et al. Comparison of frequencies of left ventricular systolic and diastolic heart failure in Chinese living in Hong Kong. Am J Cardiol 1999;84:563–7. [DOI] [PubMed] [Google Scholar]
- 2.Henein MY, Gibson DG. Long axis function in disease. Heart 1999;81:229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu CM, Sanderson JE. Right and left ventricular diastolic function in patients with and without heart failure: effect of age, sex, heart rate, and respiration on Doppler-derived measurements. Am Heart J 1997;134:426–34. [DOI] [PubMed] [Google Scholar]
- 4.European Study Group on Diastolic Heart Failure. Working Group Report: How to diagnose diastolic heart failure. Eur Heart J 1998;19:990–1003. [DOI] [PubMed] [Google Scholar]
- 5.Henry WL, De Maria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in 2-D echocardiography. Circulation 1980;62:212–17. [DOI] [PubMed] [Google Scholar]
- 6.Sahn DJ, De Maria A, Kisslo J, et al. Recommendations regarding quantitation in M mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 7.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- 8.Schiller NB. Two-dimensional echocardiographic determination of left ventricular volume, systolic function, and mass. Summary and discussion of the 1989 recommendations of the American Society of Echocardiography. Circulation 1991;84(suppl 3):I280–7. [PubMed] [Google Scholar]
- 9.Willenheimer R, Cline C, Erhardt L, et al. Left ventricular atrio-ventricular plane displacement: an echocardiographic technique for rapid assessment of prognosis in heart failure. Heart 1997;78:230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy D, Anderson KM, Savage DD, et al. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. Ann Intern Med 1988;108:7–13. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;i:307–10. [PubMed] [Google Scholar]
- 12.Gandhi SK, Powers JC, Nomeir A-M, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:17–22. [DOI] [PubMed] [Google Scholar]
- 13.Yip G, Sanderson JE. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001;344:1401. [DOI] [PubMed] [Google Scholar]
- 14.Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev 1989;69:1228–315. [DOI] [PubMed] [Google Scholar]
- 15.Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin 2000;18:435–42. [DOI] [PubMed] [Google Scholar]
- 16.Brutsaert DL. Diagnosing primary diastolic heart failure. Eur Heart J 2000;21:94–6. [DOI] [PubMed] [Google Scholar]
- 17.Caruana L, Petrie MC, Davie AP, et al. Do patients with suspected heart failure and preserved systolic functions suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ 2000;321:215–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacFadyen RJ, MacLeod CM, Shiels P, et al. Isolated diastolic heart failure as a cause of breathlessness in the community: the Arbroath Study. Eur J Heart Failure 2001;3:243–8. [DOI] [PubMed] [Google Scholar]
- 19.Sanderson JE, Chan SKW, Chan WM, et al. The aetiology of Heart failure in the Chinese population of Hong Kong – a prospective study of 730 consecutive patients. Int J Cardiol 1995;51:29–35. [DOI] [PubMed] [Google Scholar]