Abstract
The mammalian brain contains a population of neural stem cells (NSC) that can both self-renew and generate progeny along the three lineage pathways of the central nervous system (CNS), but the in vivo identification and localization of NSC in the postnatal CNS has proved elusive. Recently, separate studies have implicated ciliated ependymal (CE) cells, and special subependymal zone (SEZ) astrocytes as candidates for NSC in the adult brain. In the present study, we have examined the potential of these two NSC candidates to form multipotent spherical clones—neurospheres—in vitro. We conclude that CE cells are unipotent and give rise only to cells within the glia cell lineage, although they are capable of forming spherical clones when cultured in isolation. In contrast, astrocyte monolayers from the cerebral cortex, cerebellum, spinal cord, and SEZ can form neurospheres that give rise both to neurons and glia. However, the ability to form neurospheres is restricted to astrocyte monolayers derived during the first 2 postnatal wk, except for SEZ astrocytes, which retain this capacity in the mature forebrain. We conclude that environmental factors, simulated by certain in vitro conditions, transiently confer NSC-like attributes on astrocytes during a critical period in CNS development.
Cells isolated from the postnatal and adult brain that can grow as multipotent proliferative clones—neurospheres (1)—are capable of giving rise to neurons, astrocytes, and oligodendrocytes. Neurosphere-forming cells can be considered as neural stem cells (NSC) and can serve as a model of basic neurodevelopmental processes as well as a potential source of transplantable cells for the treatment of brain injury and neurodegenerative disease. A crucial step in understanding the biology of NSC is their identification and in vivo localization. It has been shown that the periventricular subependymal zone (SEZ) is a source of neurogenesis, and this region presumably contains the highest concentration of NSC in the postnatal and adult brain (2). Ciliated ependymal (CE) cells, lining the ventricular system subjacent to the SEZ, are another suggested source of NSC. The notion that CE cells might be capable of generating new neurons in adult mammals has long been suspected (3) and has been supported by reports that CE produce neurons after CNS injury in lower vertebrates (4, 5). However, studies in mammals have reached conflicting conclusions. Thus, it has been reported (6) that individual CE cells of rodents are NSC because they form neurospheres when cultured in isolation. In contrast, other investigators (7) found that CE cells, while capable of forming neurosphere-like clones in vitro, are unipotent because they give rise only to astrocyte-like cells. Yet, a third study (8) failed to show the formation of CE cell-derived clonal structures at all and, instead, presented evidence that a special type of astrocyte residing within the SEZ has NSC attributes. Furthermore, it has been hypothesized that a basic fibroblast growth factor (bFGF)-responsive NSC isolated from adult rat brain may in fact be a glial precursor, possibly an “astrocyte-like” cell (9). Finally, it has recently been reported that early postnatal oligodendrocyte precursor cells have NSC characteristics in vitro, but these characteristics seem to emerge only after the precursor cells are cultured in conditions that cause them to pass through a state of differentiation as type 2 astrocytes (10).
To study the identity of the NSC (11), we use clonogenic culture conditions, and a transgenic mouse that allows for the analysis of the progeny of cells within the astrocyte lineage, to examine the ability of CE cells and astrocytes to form multipotent neurospheres. We demonstrate a “global” ability for astrocytes from the cerebral cortex, cerebellum, spinal cord, and SEZ grown as monolayers to form neurospheres that can assume neuronal cell phenotypic characters when replated in suspension cultures containing the growth factors epidermal growth factor (EGF) and bFGF. CE cells cultured similarly generate spherical clones, but these clones do not display NSC characteristics in our paradigm, because they give rise only to cells with the phenotypic characteristics of glial cells. Furthermore, we show that astrocyte multipotency is restricted to early postnatal ages, except for SEZ astrocytes, which retain this ability in the mature brain.
Experimental Procedures
Isolation and Culture of CE Cells.
Cell suspensions of periventricular tissue from Imperial Cancer Research mice (7–14 days postnatal, n > 10) were prepared as previously described (12, 13). Cells were washed, resuspended in fresh medium, and plated at low density in a Petri dish. Under these conditions, CE cells represent a low percentage of all plated cells but are readily identifiable by the spinning caused by the action of their cilia. CE cells were transferred individually to microwells by collecting them with a hand-held glass microelectrode (inside diameter approximately 50 μm) attached, via PE tubing, to a 5-cc syringe. Before plating, microwells were coated with an inert, antiadhesive substance (12, 13). Cells were then cultured in N2 medium supplemented with 5% (vol/vol) FCS, EGF (10 ng/ml), and bFGF (10 ng/ml, GIBCO/BRL). Aliquots of fresh growth factor-containing medium were added every other day.
Electron Microscopy.
Ependymal cell-derived spherical clones, grown as described above, were processed for electron microscopy (EM) as previously described (12, 13). Ultrathin sections were viewed with a JEOL 2000 (Peabody, MA) transmission electron microscope. Some clones, derived from astrocyte monolayers as described below, were also processed for scanning electron microscopy by using standard procedures of fixation, critical-point drying, coating, and examination with a JSM-5900LV SEM at an acceleration voltage of 3 kV.
Generation of Monolayer Cultures.
Astrocyte monolayers (n = 36; see Table 1) were derived from the postmortem cerebral cortex, cerebellum, SEZ, and spinal cords of embryonic day 18, early postnatal (postnatal day 1–10), late postnatal (postnatal day 11–20), and adult (>20 days) ICR or Gtv-a transgenic mice. Cerebral cortex, cerebellum, spinal cord, and SEZ were dissected and processed separately to generate astrocyte monolayers. The cerebral cortex dissection was performed in such a way as to exclude all cells of the ventricular or subependymal region, accomplished by first blocking the rostral forebrain in the coronal plane, and then shaving superficial slices of the cerebrum tangential to the pial surface. Primary cultures were generated by mincing the tissue with a razor blade, incubating in trypsin, and triturating with fire-polished glass pipettes. The resulting cell suspension was seeded in a flask in DMEM/F12 medium containing 10% (vol/vol) FCS and allowed to grow to confluence. Fibroblasts were obtained from postmortem mouse muscle that was minced with a razor blade, incubated in trypsin, and triturated with a fire-polished Pasteur pipette. The resulting cell suspension was seeded in tissue culture flasks as described above to yield a highly enriched culture of fibroblasts. Microglia cultures were generated from primary monolayers derived from the brain as described above for astrocyte cultures. When the primary brain dissociate reached confluence, 20% colony stimulating factor (CSF), and dextran microbeads (Cytodex; Pharmacia) were added to the culture. After approximately 1 wk, the beads were removed and lightly vortexed to remove adherent microglia. The beads were allowed to sediment, and the cell suspension was aspirated, centrifuged, and replated in a flask containing DMEM/F12 with 20% (vol/vol) CSF and 10% (vol/vol) FCS.
Table 1.
Summary of the neurosphere-generating potential of astrocyte monolayers derived from different CNS regions at different ages
| NS+ | NS− | |
|---|---|---|
| E18 cortex | X | |
| E18 cerebellum | X | |
| E18 SEZ | X | |
| P1 spinal cord | X | |
| P1 cortex | X | |
| P2 cortex | X | |
| (5) P2 SEZ | X | |
| P3 cortex | X | |
| P3 cerebellum | X | |
| P7 cortex | X | |
| P7 spinal cord | X | |
| (2) P10 cortex | X | X |
| (2) P10 cerebellum | X | X |
| (2) P10 spinal cord | X | |
| (2) P10 SEZ | X | |
| P11 cortex | X | |
| P11 cerebellum | X | |
| P11 spinal cord | X | |
| (2) P15 cortex | X | |
| (2) P15 SEZ | X | |
| P16 cortex | X | |
| P16 SEZ | X | |
| (2) Adult cortex | X | |
| (2) Adult SEZ | X |
A variety of embryonic and postnatal ages were randomly chosen as sources for astrocyte monolayers (n = 36). These monolayers were subsequently tested for the ability to generate multipotent neurospheres, and the temporal arrangement of the results reveals striking differences in neurosphere-generating potential. Astrocytes from all regions are neurosphere-generating (NS+) until around postnatal day (P) 10 or 11 (notice that one set of P10 cerebral cortex and cerebellum cultures were NS+, whereas a different set failed to generate neurospheres (NS−). After this apparent “critical period,” astrocytes derived from the cerebral cortex, cerebellum, and spinal cord are NS−, whereas astrocytes from the SEZ do not observe this critical period and retain the ability to generate neurospheres well into adulthood. One astrocyte monolayer was generated from each age and region, unless indicated otherwise by the number in parentheses.
Generation of Neurospheres from Astrocyte Monolayers.
Confluent astrocyte monolayers were trypsinized, pelleted, and resuspended in serum-free 2× N2 medium, and counted. DF medium containing 2% methylcellulose was added 1:1 to the cells, which were then plated at a density of 1000 cells/cm2 in 6-well plates coated with the antiadhesive as previously described (12). Cultures were supplemented with 10 ng/ml of EGF and bFGF every 2–3 days.
BrdUrd Labeling.
To label proliferating cells, BrdUrd (10 μg/ml) was added to suspension cultures for 24 h. BrdUrd-labeled cells were detected as previously described (12, 13).
Immunocytochemistry.
Clones were placed into a drop of N2 medium containing 1% FCS on glass coverslips that had been coated sequentially with poly(L-ornithine) (10 μg/ml) and laminin (5 μg/ml). One to two days after plating on coverslips, cells were fixed with 4% paraformaldehyde in PBS for 20 min and processed as previously described (12, 13) for immunocytochemistry with antibodies against the following antigens: glial fibrillary acidic protein (GFAP), an astrocyte-specific intermediate filament protein (Immunon, Pittsburg, PA); vimentin and nestin, respective cytoskeletal markers of immature astrocytes and neural stem cells (Development Studies Hybridoma Bank, Iowa City); β-III tubulin, a neuron-specific intermediate filament protein (Promega or Sigma); L1, a cell adhesion molecule associated with neurons (a gift of M. Schachner); microtubule associated protein (MAP)-2, a microtubule protein associated with neurons (Sigma); and A2B5, a surface protein associated with O-2A progenitors capable of giving rise to either astrocytes or oligodendrocytes (Boehringer Mannheim). Some cells were counterstained with propidium iodide before viewing.
Infection of GFAP-Tva Astrocytes, and Alkaline Phosphatase Histochemistry.
Astrocyte monolayers were prepared as described above from the cerebral cortices of P2 Gtv-a transgenic mice (14). Avian leukosisvirus, containing either a LacZ or alkaline phosphatase insert as a marker, was secreted by cultured chicken fibroblasts, and infection of astrocytes was accomplished by continuously incubating Gtv-a astrocytes in a medium conditioned by the fibroblasts.
Results
The ventricular system of the mouse is lined with a single layer of CE cells. By dissecting and mechanically dissociating the P1–14 periventricular region, we are able to generate a single-cell suspension containing CE cells that are readily identifiable by their hypermotility caused by the rapid beating of cilia. Using phase optics, it is possible to visualize individual CE cells (Fig. 1a, upper left Inset) and suction them into a glass microelectrode for transport to individual microwells where approximately 20% (n > 200 from five mice) grow into spherical clones (Fig. 1). We added EGF and bFGF to promote neurosphere production as we (12, 13) and others (1) previously used to stimulate the production of multipotent neurospheres from the SEZ and hippocampus. After a few days in growth factor-supplemented medium, phase-bright masses become apparent in individual microwells and continue to increase in size for several days (Fig. 1a, lower right Inset). These phase-bright masses are revealed to be cellular clones because, on removal from microwells and plating on polyornithine/laminin coated coverslips, they quickly attach and begin to differentiate. The clones flatten as cells begin migrating and extending processes away from the central core, and immunocytochemistry reveals that all of these cells express GFAP (Fig. 1a). Progeny of clones derived from CE cells never exhibit immunoreactivity for β-III tubulin, even though this marker consistently labels both immature and mature neurons derived from neurospheres obtained from whole SEZ dissociates by using identical culture and labeling methods (data not shown). Additionally, electron microscopy reveals that at least some of the cells within these CE-derived spheres maintain ependymal cell phenotype as indicated by extensive ciliation (Fig. 1b).
Figure 1.
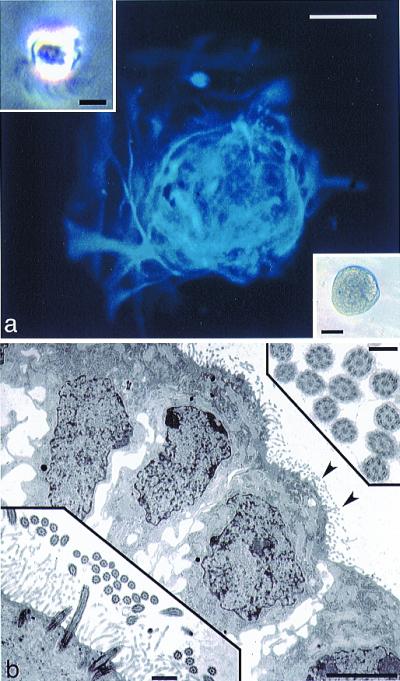
Generation of spherical clones from single, ciliated ependymal cells. (a) GFAP immunofluorescence (blue, AMCA) of a clone (phase microscopy of such a clone is shown in the lower right Inset), derived from a single ciliated ependymal cell (Inset, upper left; notice cilia protruding from the base of the cell). Scale bar in a and lower right Inset = 50 μm; scale bar in upper left Inset = 5 μm. (b) TEM of a clone derived from a single ciliated ependymal cell. Note cilia on the surfaces of cells at the edge of this clone (arrowheads), shown at higher magnification in the lower left Inset. The upper right Inset shows cross-sectioned cilia displaying the characteristic 9 + 2 arrangement of microtubules. Scale bar in b = 5 μm, 0.5 μm in the lower left Inset, and 0.2 μm in the upper right Inset.
To examine the potential for astrocytes to display NSC characteristics, we first generated monolayer cultures of astrocytes from the cerebral cortex, cerebellum, spinal cord, and SEZ from mice ranging in age from late embryonic to adult. The primary fraction of cells used to generate astrocyte monolayers initially contains cells that are immunopositive for a variety of phenotypic antigens, including the oligodendrocyte protein O4, and neuron-specific β-III tubulin (data not shown). However, after 7–10 days in vitro, plated cells form a confluent monolayer (Fig. 2a), and these markers fail to label any cells. At this stage, a majority of cells in the monolayer are immunopositive for the astrocyte-specific intermediate filament protein GFAP (Fig. 2b). However, because some of the cells in the monolayer are weakly labeled by, or do not express, this marker, we also show that an even greater percentage (95–100%) of the monolayer cells are immunopositive for other markers of immature and reactive astrocytes, including vimentin (Fig. 2c), as well as nestin (not shown) and S100β (Fig. 2c Inset). Within the primary fraction, the only other cells that we are able to immunologically identify are microglia, which express the antigen macrophage-1 (Mac-1) (Fig. 2a, lower right Inset). The percentage of Mac-1-positive cells present in the primary fraction typically ranges from 1–5%, and Mac-1 immunostaining is essentially abolished by passaging, leaving only cells with astrocytic morphology and antigen expression. Monolayer cultures are immunonegative for collagen types I and IV, and Von Willebrand's Factor, indicating that there is no detectable contamination either by fibroblasts or endothelial cells (data not shown).
Figure 2.
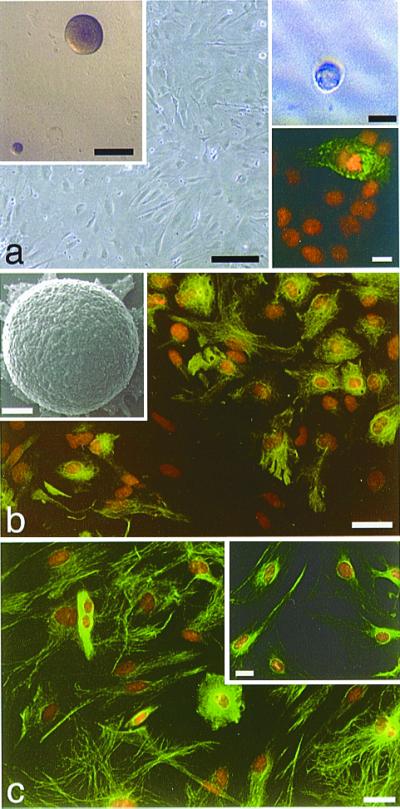
Astrocyte monolayers from P2 mouse cerebral cortex generate spherical clones. (a) Phase micrograph of a confluent layer of astrocytes. Scale bar = 50 μm. Early passage monolayers often contain a small number of microglia, as seen in the lower right Inset using immunofluorescence for Mac-1 (FITC, green), counterstained with propidium iodide (PI, orange). Scale bar in lower right Inset = 10 μm. Astrocyte monolayers from this age also generate spherical clones when grown in suspension culture with EGF and bFGF (upper left Inset, Scale bar = 100 μm; and Inset in b, showing an SEM of such a clone; Scale bar = 50 μm). Similar clones are generated when astrocytes are cultured in isolation (phase micrograph of clone derived from a single astrocyte is shown in the upper right Inset, Scale bar = 30 μm). (b) GFAP immunolabeling (green) and PI counterstaining of most, but not all, astrocytes within the monolayer. Scale bar = 25 μm. (c) Clones are generated from monolayers such as these in which virtually all cells within the monolayer exhibit immunolabeling for the immature astrocyte markers, vimentin (green, PI counterstaining) and S100β (Inset). Scale bar = 25 μm in c and 10 μm in the Inset.
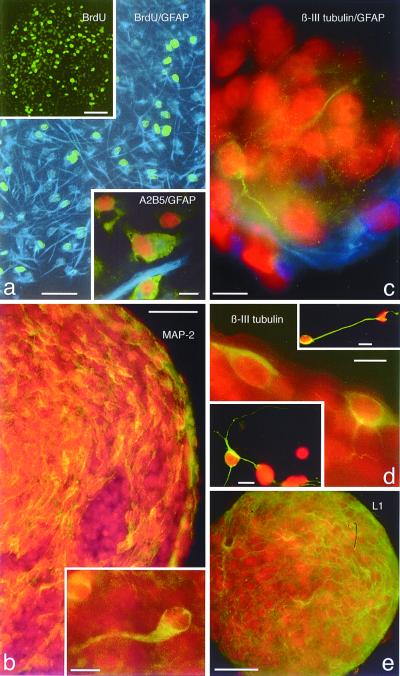
When astrocyte monolayers are detached and grown as suspension cultures in the presence of EGF and bFGF (12), between 1 and 10% of the cells generate spherical clones (Fig. 2a, upper left Inset). We believe that these structures are composed of clonally related cells and do not simply represent cellular aggregates, because of the following: first, we plated dissociated astrocytes in semisolid methylcellulose medium that hinders cell motility; second, they have a sharp, continuous outer border unlike that seen with cellular aggregates; third, we do not see these structures when growth factors are omitted; fourth, the addition of BrdUrd to the culture medium can label virtually all of the cells within a sphere, indicating that each cell is newly generated (Fig. 3a and upper left Inset); and fifth, it is possible to generate similar structures when single astrocytes are cultured individually in microwells (Fig. 2a, upper right Inset). When removed from suspension culture and plated onto polyornithine/laminin coated glass, astrocyte-derived spheres readily attach, and the cells at the bottom begin to migrate, differentiate, and send out processes (Fig. 2b Inset and Fig. 3). The largest percentage of sphere cells are immunopositive for GFAP (Fig. 3a and Inset); however, many cells are labeled for the O-2A marker A2B5 (Fig. 3a lower Inset), as well as the neuron-specific markers β-III tubulin, L1, and microtubule-associated protein 2 (MAP-2; Fig. 3 b–e). It should be noted that these results are obtained with astrocyte monolayers even after five passages (the highest passage tested), but it is our impression that there is frequently a reduction in neurosphere yield with increasing passages. Also, we have found that primary neurospheres can generate secondary neurospheres after dissociation, indicating that these astrocyte-derived neurospheres can be passaged and exhibit self-renewal.
Figure 3.
Astrocyte-derived neurospheres are proliferative and express neuronal and glial antigens. (a) BrdUrd (green) and GFAP (blue) immunofluorescence double-labeling of a sphere derived from a P2 cortical astrocyte monolayer, showing the highly proliferative nature of these sphere cells. BrdUrd was present in the culture medium for 24 h before plating. Scale bar = 50 μm. Upper left Inset: Low magnification BrdUrd immunofluorescence labeling of an entire, attached, differentiating neurosphere. Scale bar = 50 μm. Lower right Inset shows double immunolabeling for A2B5 (green) and GFAP (blue), PI counterstained, of differentiating cells from an astrocyte-derived neurosphere. Scale bar = 10 μm. (b) Immunolabeling of a single P7 cerebellar astrocyte-derived neurosphere showing MAP-2 positive cells, indicating the presence of neurons within these clones. The Inset in b shows a higher magnification of a single MAP-2-positive immature neuron within the neurosphere. Scale bars = 30 μm in b, and 10 μm in the Inset. (c) GFAP (blue) and β-III tubulin (green), PI counterstained, double immunolabeling of a neurosphere derived from a P1 cerebral cortex astrocyte monolayer. Note the beta-III tubulin immunopositive neurites arborizing around PI-stained cells, and GFAP astrocytic processes within the lower portion of the neurosphere. Scale bar = 10 μm. (d) β-III tubulin immunolabeling of a neurosphere derived form a P1 spinal cord astrocyte monolayer. Note two β-III tubulin-positive immature neurons at the edge of the neurosphere, compared with apparently more mature immunolabeled neurons (upper right and lower left Insets) that have migrated away from the neurosphere and elaborated long processes. Scale bars = 10 μm in d and both Insets. (e) L1 immunolabeling (green, PI counterstaining) of a neurosphere from a P1 spinal cord astrocyte monolayer, showing pervasive labeling of neurons throughout the neurosphere. Scale bar = 25 μm.
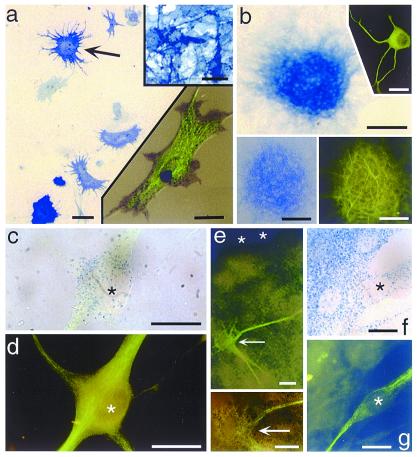
To rule-out that cells other than astrocytes are responsible for generating the neurospheres, we tested fibroblasts and microglia in our suspension culture paradigm and found that neither cell forms neurospheres under these conditions. This control experiment strongly suggests that the cells giving rise to multipotent neurospheres are, in fact, astrocytes; however, to get a final, positive confirmation of the astrocytic identity of neurosphere-forming cells, we exploited the transgenic Gtv-a mouse that contains the tv-a gene encoding the TVA receptor for avian leukosisvirus under control of the GFAP promoter, which allows for the selective infection of GFAP-expressing astrocytes with the avian leukosisvirus (RCAS). When using an RCAS with a reporter gene, e.g., alkaline phosphatase (AP), it is possible to identify positively all of the progeny of a single infected astrocyte. Once the RCAS is internalized and incorporated into the infected cell's genome, it becomes constitutively expressed; therefore, all progeny of an infected cell will express the marker gene, even if they do not themselves express GFAP. We derived astrocyte monolayers from Gtv-a mice and cultivated these monolayers in a medium conditioned by chicken embryo fibroblasts genetically modified to produce RCAS-AP (14). Under these conditions, a small percentage of astrocytes within the monolayer (infection rate in this study varied from 1–16%) become infected with RCAS-AP, as indicated by histochemical detection of AP (Fig. 4a and lower Inset). When such astrocyte monolayers are replated and grown in suspension culture, some of the resulting clones are composed of AP-expressing cells (Fig. 4 b and e), indicating that GFAP-positive astrocytes are capable of forming the neurospheres seen in our culture paradigm. Furthermore, subsequent immunolabeling of neurospheres derived from a single RCAS-infected astrocyte reveals the presence of cells within (Figs. 4b Insets, 4e and Inset) and outside (Fig. 4 c, d, f, and g) the neurospheres that express neuron phenotype markers, including MAP-2 (Fig. 4b lower right Inset) and neuronal beta III tubulin (see Figs. 4b Inset and c–g); 14 RCAS-AP-infected neurospheres were analyzed, of which 13 exhibited variable numbers of β-III tubulin or MAP-2 positive neurons. Identical results as documented in Fig. 4 also were obtained when using an RCAS containing the LacZ reporter gene (data not shown).
Figure 4.
Alkaline phosphatase (AP) enzyme histochemistry and immunocytochemistry of cells and neurospheres derived from Gtv-a transgenic mouse astrocytes infected with RCAS-alkaline phosphatase, showing cells expressing both AP and neuronal phenotype markers. (a) P2 astrocyte monolayer after infection with the avian leukosisvirus expressing the AP reporter gene, showing infected astrocytes (e.g., arrow). Upper right Inset shows AP histochemistry of the DF1 chicken embryo fibroblast line engineered to produce the RCAS-AP leukosisvirus. Lower right Inset shows a single infected astrocyte with both AP histochemical labeling (blue-black punctae) and GFAP immunofluorescence (green, FITC). Scale bars = 25 μm in a, 30 μm in both Insets. (b) A neurosphere derived from a Gtv-a astrocyte monolayer. AP histochemistry reveals cells of this neurosphere expressing the RCAS-AP gene, thus indicating derivation of the clone from a single, infected astrocyte. Upper right Inset shows an example of a neuron derived from such a neurosphere, immunofluorescence for β-III tubulin (green, FITC). Lower pair of Insets show the same neurosphere expressing AP (left) and MAP-2 (right), revealing numerous MAP-2-positive processes emanating from an RCAS-infected neurosphere. Scale bars = 100 μm in b, 50 μm in the lower Insets, and 40 μm in the upper Inset. (c and d) A single RCAS-AP infected neuron that has migrated away from its neurosphere and differentiated. Brightfield labeling of AP reaction product (blue dots) in this neuron (c), colocalized with β-III tubulin immunofluorescence (FITC green) shown in d. Asterisk marks the nucleus of this cell in each figure. Scale bars in c and d = 10 μm. (e) Low (e) and high (lower Inset) magnification of an RCAS-AP-infected neurosphere showing β-III tubulin-positive neurons (FITC, green) within a densely AP-positive neurosphere. Arrow points to double labeled neurons within the neurosphere. Asterisks mark the top edge of the neurosphere. Scale bar = 10 μm. (f and g) A double labeled neuron at the edge of a neurosphere (the neurosphere is in the upper left portion of the field), as seen in single exposures of the same field, brightfield for AP in f and immunofluorescence for β-III tubulin in g. The asterisks are within the nucleus of this double labeled cell in both images, and other nuclei appear as sparsely positive AP ovals. Scale bars = 10 μm.
The ability of astrocyte monolayers to produce spherical clones is discretely age and region related (see Table 1). When we compare the spatiotemporal characteristics of the tissues from which the astrocyte monolayers were derived, there is a strikingly abrupt failure for astrocyte monolayers derived from the cerebral cortex, cerebellum, and spinal cord to generate any neurospheres from animals older than postnatal day 10/11. For cultures of astrocytes produced before P10/11, there are variable numbers of neurospheres generated, ranging from 100–1,000 per 10,000 astrocytes plated. We failed to detect any neurospheres from cultures derived from animals older than P10/11. In contrast, SEZ-derived astrocytes continue to produce multipotent clones even when generated from adult animals.
Discussion
We conclude that astrocytes globally possess NSC attributes during embryonic and early postnatal development. These attributes disappear near the end of the second postnatal week, except for SEZ astrocytes, which continue to form neurospheres even when derived from adult animals. Additionally, we show that CE cells can form spherical clones, but that these clones give rise only to astrocyte-like cells. It should be noted that, while we confirm the findings of Chiasson and colleagues (7) regarding the limited potency of CE cells, we did not use exactly the same culture methods used in the study of Johansson and colleagues (6) showing that CE cells can form neurospheres, and therefore are neural stem cells. However, it is clear that our culture conditions—sufficient for stimulating the production of multipotent neurospheres from single astrocytes—stimulate from single CE cells the production only of unipotent clones. Why there should be this differential effect is not known.
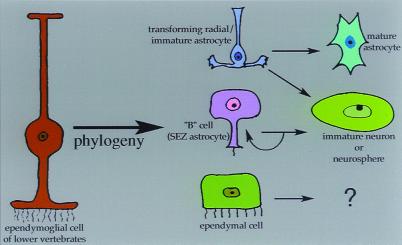
Our results—combined with previous studies on the biology of neural stem cells and the phylogenesis and ontogenesis of glia—lead us to propose a model (Fig. 5) in which the neurosphere-generating cells in astrocyte monolayers derived from young animals might represent relatively immature astrocytes (15), possible transitioning radial glia that have not yet completely assumed the genetic program of mature astrocytes (16). At or around P11, some widespread event occurs that causes astrocytes to lose the ability, at least in our paradigm, to display NSC attributes. SEZ-derived astrocytes, however, retain their NSC characteristics even after this “critical period,” suggesting that factors within the SEZ act to preserve the neurosphere-forming ability of astrocytes into adulthood. The notion that cells of the radial glial lineage might have attributes of stem cells at some time during development is not new, with studies implicating this transient glial morphotype as a possible NSC during both embryonic¶ (17) and postnatal (19, 20) development. The present study did not directly test for the ability of radial glia to generate neurospheres; however, a recent preliminary report¶ has described the ability of isolated embryonic radial glia to generate neurons. The restricted time-course of neurosphere-generating ability by cells in our cultures, occurring only from monolayers derived from embryonic and early postnatal CNS, is strikingly similar to the time-course of radial glial transformation into astrocytes seen in vivo in rodents (16, 21); furthermore, a phylogenetic lineage relationship between ependymal cells and radial glia has been put forth (22), and studies have shown that ependymal cells of some lower vertebrates (5, 22), which are capable of the de novo generation of neurons postnatally, also maintain a radial morphology and perform many of the functions (neuroblast guidance, phagocytosis, and structural repair after injury) that are subserved by radial glia and astrocytes in mammals. Our model is further supported by other studies showing, for instance, that the SEZ maintains an “immature” level of extracellular matrix expression throughout adulthood (23), and that P11 is close to the time in mouse development when radial glia cease to be immunologically detectable (21) and have, for the most part, finished their morphological and biochemical transition into “mature” astrocytes near the end of a critical period when maturing astrocytes down-regulate their normal expressions of boundary molecules (24), and then contribute to a neurite-growth inhibitory environment after injury (25). Additionally, the transition from radial to stellate glial morphology has been shown to be bidirectional (16, 26), with first postnatal week astrocytes responding to a diffusible factor produced by E14 radial glia by reassuming a radial morphology and reexpressing the radial glial marker RC2, while down-regulating the astrocyte marker GFAP. It may be that the neurosphere-generating cells from our monolayer culture represent a “radial” phenotype that is functionally related to the multipotent SEZ B cell astrocyte described by Alvarez-Buylla and colleagues (8, 27). Radial glia differentiate into mature astrocytes that are incapable of forming multipotent clones in vitro, whereas the B cell astrocyte is maintained in a multipotent state, due perhaps to the persistent expression of extracellular matrix (ECM) and other developmentally regulated molecules (23). Why, then, does postnatal and adult neurogenesis seem to be restricted primarily to the periventricular germinal zone? Because early postnatal cortical radial glia or astrocytes share neurosphere-generating potential with the SEZ B cells, then why don't these multipotent radial cells produce neurons in other regions besides the SEZ? We don't know, but it could be that, to display NSC characteristics in vivo, a cell requires specific environmental factors. Indeed, human fetal NSC can survive and differentiate into neurons only when grafted to the embryonic and newborn mouse brain while the essential cues still persist, but not in the normal mature brain where they may be lost (18, 28). Such factors or specific cell–cell interactions present within the SEZ [as suggested by Doetsch and colleagues (8), who showed that many of the neurogenic B cell astrocytes of the SEZ maintain a connection with the ventricular surface (but so do ependymal cells; their demonstrated lack of multipotency could relate to distinct cell characteristics, e.g., their being in a more differentiated state relative to SEZ B cells)] may be mimicked by our culture conditions that either direct or allow astrocytes to display multipotency.
Figure 5.
A theoretical model of roles for glial subtypes in the postnatal generation of neurons and neurospheres. Using our tissue culture paradigm, ependymal cells can generate unipotent spherical clones but do not give rise to neurons or neurospheres. However, the generation of multipotent clones from ependymal cells, under distinct culture conditions, has been described (6). The subependymal zone (SEZ) B cell has been shown to display neural stem cell characteristics (8), and the SEZ B cell may be the cell responsible for neurosphere generation from SEZ-derived astrocyte monolayers. We believe that the neurosphere-generating cell in astrocyte monolayers from non-SEZ regions is another type of astrocyte, possibly a transforming radial glial cell that shares, transiently, the neural stem cell characteristics of the SEZ B cell astrocyte; this cell loses these characteristics after its transformation into a mature astrocyte, as has been shown to occur in the first to second postnatal weeks in rodents (21). All of these putative multipotent cells may be phylogenetically related to the ependymoglial cell of invertebrates (22), which performs all of the functions subserved by mammalian ependymal cells, radial glial cells, and astrocytes.
In conclusion, the findings presented here support the idea that morphologically differentiated astrocytes may serve as multipotent cells with NSC-like attributes, suggesting that a “de-differentiation” event occurs under particular tissue culture conditions that endows astrocytes with more primitive ontogenetic characteristics, specifically, the ability to generate neurons and glia. An important remaining question for restorative neurology is whether or not mature astrocytes, perhaps even from the aged brain, can again be induced to exhibit NSC characteristics. Along this line, preliminary studies in which we pretreated postcritical period astrocytes with growth factors resulted in the generation of large numbers of neurospheres, strongly suggesting that conditions can be created that may de-differentiate chronologically older astrocytes in such a way as to reinstate NSC-like characteristics.
Acknowledgments
We thank Dr. Fitzgerald for the SEM images, and Dr. Walker for advice on microglia cultures. Some antibodies were obtained from the Development Studies Hybridoma Bank. This work was supported by the Spinal Cord Research Foundation, Paralyzed Veterans of America (E.D.L.) and by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants NS37556 (D.A.S.) and NS14841 (P.R.).
Abbreviations
- CNS
central nervous system
- SEZ
subependymal zone
- NSC
neural stem cell
- CE
ciliated ependymal cell
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- GFAP
glial fibrillary acidic protein
- MAP-2
microtubule-associated protein 2
- Mac-1
macrophage-1
- AP
alkaline phosphatase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250471697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250471697
Malatesta, P., Hartfuss, E. & Gotz, M. (1999) Soc. Neurosci. Abstr. 25, 522 (abstr.).
References
- 1.Reynolds B A, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S, Dunne C, Hewson J, Wohl C, Wheatly M, Peterson A C, Reynolds B A. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman J. Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M J, Waxman S G. Ann N Y Acad Sci. 1985;457:213–233. doi: 10.1111/j.1749-6632.1985.tb20807.x. [DOI] [PubMed] [Google Scholar]
- 5.Molowny A, Nacher J, Lopez-Garcia C. Neuroscience. 1995;68:823–836. doi: 10.1016/0306-4522(95)00201-s. [DOI] [PubMed] [Google Scholar]
- 6.Johansson C B, Momma S, Clarke D L, Risling M, Lendahl U, Frisén J. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 7.Chiasson B J, Tropepe V, Morshead C M, van der Kooy D. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doetsch F, Caille I, Lim D A, Garcia-Verdugo J M, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 9.Palmer T D, Markakis E A, Willhoite A R, Safar F, Gage F H. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo T, Raff M. Science. 2000;289:1754–1756. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 11.Scheffler B, Horn M, Blumcke I, Laywell E D, Coomes D, Kukekov V G, Steindler D A. Trends Neurosci. 1999;22:348–357. doi: 10.1016/s0166-2236(99)01416-2. [DOI] [PubMed] [Google Scholar]
- 12.Kukekov V G, Laywell E D, Thomas L B, Steindler D A. Glia. 1997;21:399–407. doi: 10.1002/(sici)1098-1136(199712)21:4<399::aid-glia7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 13.Kukekov V G, Laywell E D, Suslov O, Davies K, Scheffler B, Thomas L B, O'Brien T F, Kusakabe M, Steindler D A. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 14.Holland E C, Varmus H E. Proc Natl Acad Sci USA. 1998;92:2061–2065. [Google Scholar]
- 15.Levitt P, Rakic P. J Comp Neurol. 1980;193:815–40. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 16.Cameron R S, Rakic P. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- 17.Frederiksen K, McKay R D G. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flax J D, Aurora S, Yang C H, Simonin C, Wills A M, Billinghurst L L, Jendoubi M, Sidman R L, Wolfe J H, Kim S U, Snyder E Y. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Buylla A, Theelen M, Nottebohm F. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 20.Gray G E, Sanes J R. Development. 1992;114:271–283. doi: 10.1242/dev.114.1.271. [DOI] [PubMed] [Google Scholar]
- 21.Misson J-P, Takahashi T, Caviness V S. Glia. 1991;4:138–148. doi: 10.1002/glia.440040205. [DOI] [PubMed] [Google Scholar]
- 22.Reichenbach A, Robinson S R. In: Neuroglial Cells. Ransom B R, Kettenman H, editors. Oxford: Oxford Univ. Press; 1994. pp. 58–84. [Google Scholar]
- 23.Gates M A, Thomas L B, Howard E M, Laywell E D, Sajin B, Faissner A, Gotz B, Silver J, Steindler D A. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 24.Cooper N G F, Steindler D A. Brain Res. 1989;489:167–176. doi: 10.1016/0006-8993(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 25.Rudge J S, Smith G M, Silver J. Exp Neurol. 1989;103:1–16. doi: 10.1016/0014-4886(89)90180-5. [DOI] [PubMed] [Google Scholar]
- 26.Hunter K E, Hatton M E. Proc Natl Acad Sci USA. 1995;92:2061–2065. doi: 10.1073/pnas.92.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doetsch F, Garcia-Verdugo J M, Alvarez-Buylla A. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brustle O, Choudhary K, Karram K, Huttner A, Murray K, Dubois-Dalcq M, McKay R D G. Nat Biotechnol. 1998;16:1040–1044. doi: 10.1038/3481. [DOI] [PubMed] [Google Scholar]