Abstract
Background: Conventional Doppler indices of left ventricular diastolic function do not correlate with symptoms or exercise capacity in patients with hypertrophic cardiomyopathy, because of their dependence on loading conditions. Diastolic mitral annular velocity measured using Doppler tissue imaging has been reported to be a preload independent index of left ventricular diastolic function.
Objective: To determine the relation between diastolic annular velocities combined with conventional Doppler indices and symptoms or exercise capacity in hypertrophic cardiomyopathy.
Methods: 85 patients with hypertrophic cardiomyopathy and 60 normal controls were studied. Diastolic mitral annular velocities, transmitral left ventricular filling, and pulmonary venous velocities were measured.
Results: Early diastolic velocities at lateral and septal annulus were lower in patients with hypertrophic cardiomyopathy than in controls (lateral Ea: 10 (3) v 18 (4) cm/s, p < 0.0001; septal Ea: 7 (2) v 12 (3) cm/s, p < 0.0001). Unlike conventional Doppler indices alone, transmitral early left ventricular filling velocity (E) to lateral Ea ratio correlated inversely with peak oxygen consumption (r = −0.42, p < 0.0001). Patients in New York Heart Association (NYHA) class III had a higher transmitral E to lateral Ea ratio (12.0 (4.6)) than those in NYHA class II (7.6 (3.1), p < 0.005) or class I (6.6 (2.6), p < 0.0001).
Conclusions: Early diastolic mitral annular velocities are reduced in patients with hypertrophic cardiomyopathy. Unlike conventional Doppler indices alone, the transmitral E to lateral Ea ratio correlates with NYHA functional class and exercise capacity.
Keywords: Doppler tissue imaging, mitral annular velocity, hypertrophic cardiomyopathy
Many of the clinical and pathophysiological features of hypertrophic cardiomyopathy result from a complex disturbance of diastolic function.1–10 Although transmitral left ventricular filling velocities recorded by Doppler echocardiography are widely used to assess left ventricular diastolic function, most studies have failed to show significant correlations between Doppler derived transmitral velocities and the severity of left ventricular hypertrophy, symptoms, exercise capacity, or mean left atrial pressure in patients with hypertrophic cardiomyopathy.6, 7, 9, 11, 12 Conventional echocardiographic Doppler indices are unreliable for assessing left ventricular diastolic function in such patients, probably because of their dependence on loading conditions.13, 14
Doppler tissue imaging, a new echocardiographic application recently developed for clinical use, has made possible the acquisition of myocardial wall and annular velocities on-line during ultrasound examination.15, 16 Early diastolic mitral annular velocity measured using Doppler tissue imaging has recently been reported to be a preload independent index for evaluating left ventricular diastolic function.12, 17–19
Our aim in this study was to determine the relation of diastolic mitral annular velocities combined with conventional Doppler indices to the degree of hypertrophy, symptoms, or exercise capacity in hypertrophic cardiomyopathy.
METHODS
Patients
Eighty five consecutive patients (age 10–72 years; mean (SD) age, 38 (14) years; 56 male, 29 female) with hypertrophic cardiomyopathy were studied prospectively. The diagnosis of hypertrophic cardiomyopathy was based on the echocardiographic demonstration of unexplained left ventricular hypertrophy.20 Patients were selected by the following criteria: normal sinus rhythm; heart rate < 90 beats/min at the time of Doppler tissue imaging study; absence of moderate to severe mitral regurgitation; left ventricular ejection fraction > 50%; and normal left ventricular cavity dimensions. Cardioactive drug treatment was discontinued for at least five half lives before Doppler tissue imaging studies, except in 22 patients who continued to take amiodarone. The study cohort was compared with 60 age matched controls (age 9–69 years; mean (SD) age 37 (16) years; 30 male, 30 female) without signs or symptoms of heart disease.
Echocardiography
Imaging was done in the left lateral decubitus position using an Acuson 128 XP/10 (Mountain View, California, USA) with a multifrequency transducer equipped with Doppler tissue imaging software. Standard views for M mode and cross sectional studies were obtained. Standard techniques were employed for sizing the left ventricle and left atrium. The magnitude and distribution of left ventricular hypertrophy were assessed in the parasternal short axis plane by dividing the ventricle into four regions: anterior septum, posterior septum, lateral wall, and posterior wall. Wall thickness was measured at the levels of the mitral valve and the papillary muscles in each of the four segments.21, 22 Maximum left ventricular wall thickness was defined as the greatest thickness in any single segment. A semiquantitative point score of left ventricular hypertrophy (Wigle score) was calculated using a previously described method.5 Peak left ventricular outflow tract flow velocity was determined using continuous wave Doppler, and pressure gradients were calculated using the simplified Bernoulli equation. Transmitral left ventricular filling velocities at the tips of the mitral valve leaflets were obtained from the apical four chamber view using pulsed wave Doppler echocardiography. The transmitral left ventricular filling signal was traced manually and the following variables derived: peak velocity of early (E) and late (A) filling, E wave deceleration time, and E/A ratio. Isovolumetric relaxation time was determined using continuous wave Doppler echocardiography in accordance with standard methodology.23 Pulmonary venous flow signals were recorded from the right upper pulmonary vein using pulsed Doppler with colour Doppler guidance. Peak velocity of systolic, diastolic, and atrial reversal signals were recorded.
Doppler tissue imaging
From the apical four chamber view, a 10 mm Doppler sample volume was placed at the lateral and septal margins of the mitral annulus. Care was taken to align the echo image so that the annular motion was parallel to the Doppler tissue imaging cursor. A Doppler velocity range of −30 to 30 cm/s was selected using the lowest wall filter settings and the minimum optimal gain. Doppler tissue imaging velocities were recorded at a sweep speed of 100 mm/s and stored on S-VHS videotape for later playback and analysis. All measurements were made in three cardiac cycles and averaged by one investigator. The following measurements were made from the Doppler tissue imaging recordings: early (Ea) and late (Aa) diastolic velocities, and deceleration time derived by linear extrapolation of Ea to baseline. The ratio of transmitral early left ventricular filling velocity (E) to early diastolic Doppler tissue imaging velocity of the mitral annulus (transmitral E/Ea) was calculated. This ratio has been reported to correlate with left ventricular filling pressure.12, 18
Metabolic exercise testing
Eighty three patients underwent symptom limited exercise tests on an upright bicycle ergometer using a ramp protocol with simultaneous respiratory gas analysis and blood pressure recording.24 All exercise tests were performed within three to five days of the echocardiographic examination. Peak oxygen consumption (PVo2) was defined as the mean of the highest values obtained over the last 10 seconds of exercise.
Statistical analysis
Data are expressed as mean (SD). Group data were compared using the unpaired Student's t test or analysis of variance (ANOVA) with Fisher's PLSD test where appropriate. Linear regression analysis was used to compare continuous variables. A probability value of p < 0.05 was considered significant.
RESULTS
Patient characteristics
Forty three patients (51%) had dyspnoea (New York Heart Association (NYHA) functional class II (n = 37) and III (n = 6)), 24 (28%) had exertional chest pain, 32 (38%) had a history of unexplained syncope, and 19 (22%) had a history of palpitations. Nineteen patients (22%) had a family history of hypertrophic cardiomyopathy, and 23 (27%) had a family history of hypertrophic cardiomyopathy and premature (< 40 years old) sudden cardiac death. Maximum left ventricular wall thickness was 21 (4) mm (range 15–36 mm). The pattern of left ventricular hypertrophy was asymmetrical in 69 patients (81%), concentric in 12 (14%), and distal in three (4%). Left ventricular hypertrophy was confined to the posterior ventricular septum in one patient (1%). Twenty three patients (27%) had a resting left ventricular outflow tract gradient of more than 30 mm Hg.
Conventional echocardiographic findings in hypertrophic cardiomyopathy and controls
Conventional echocardiographic variables in patients with hypertrophic cardiomyopathy and controls are shown in table 1. There were no significant differences in mean transmitral E and A wave velocities between patients and controls. Technically adequate pulmonary venous flow signals were detected in 56 patients with hypertrophic cardiomyopathy and in 48 controls. Peak systolic and atrial reversal velocities of pulmonary venous flow were higher in patients with hypertrophic cardiomyopathy than in controls. Peak diastolic velocity of pulmonary venous flow was lower in patients with hypertrophic cardiomyopathy than in controls.
Table 1.
Cross sectional and Doppler echocardiographic findings in hypertrophic cardiomyopathy and controls
| Variable | HCM | Control | p Value |
| Cross sectional echocardiography | |||
| LV dimensions | |||
| End diastole (mm) | 42 (5) | 45 (4) | <0.001 |
| End systole (mm) | 23 (5) | 27 (4) | <0.0001 |
| Fractional shortening (%) | 46 (8) | 41 (5) | <0.0001 |
| Thickness | |||
| IVS (mm) | 18 (5) | 9 (1) | <0.0001 |
| Posterior wall (mm) | 11 (3) | 9 (1) | <0.0001 |
| LA dimension (mm) | 42 (6) | 33 (5) | <0.0001 |
| Doppler echocardiography | |||
| LV filling flow | |||
| Transmitral E (cm/s) | 71 (21) | 75 (15) | NS |
| Transmitral A (cm/s) | 55 (23) | 49 (13) | NS |
| E/A ratio | 1.5 (0.7) | 1.6 (0.6) | NS |
| E deceleration time (ms) | 215 (69) | 145 (30) | <0.0001 |
| Isovolumic relaxation time (ms) | 90 (19) | 72 (12) | <0.0001 |
| Pulmonary venous flow | |||
| Peak systolic velocity (cm/s) | 56 (14) | 50 (11) | <0.05 |
| Peak diastolic velocity (cm/s) | 43 (10) | 51 (14) | <0.01 |
| Peak atrial systolic velocity (cm/s) | 30 (10) | 21 (6) | <0.0001 |
Values are mean (SD).
A, late left ventricular filling velocity; E, early left ventricular filling velocity; HCM, hypertrophic cardiomyopathy; IVS, interventricular septum; LA, left atrial; LV, left ventricular.
Doppler tissue imaging in hypertrophic cardiomyopathy and controls
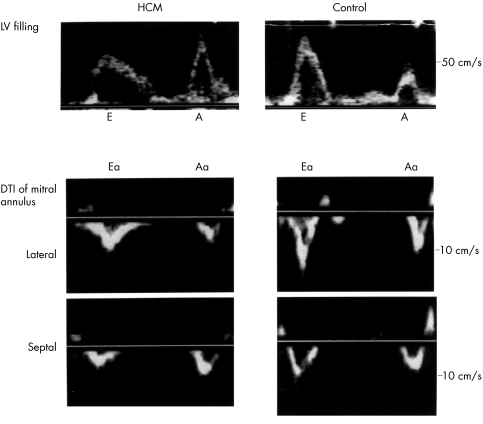
Representative examples of lateral and septal mitral annular velocities measured using Doppler tissue imaging and transmitral left ventricular filling velocities in patients with hypertrophic cardiomyopathy and controls are shown in fig 1. Mitral annular velocities in patients with hypertrophic cardiomyopathy and controls are shown in table 2. Early diastolic annular velocities were lower in patients with hypertrophic cardiomyopathy than in controls.
Figure 1.
Representative examples of lateral and septal mitral annular velocities measured using Doppler tissue imaging (DTI) and transmitral left ventricular (LV) filling velocities in patients with hypertrophic cardiomyopathy (HCM) and controls. Diastolic annular velocities showed two waves away from the apex during early diastole and atrial contraction. A, transmitral late left ventricular filling velocity; Aa, late diastolic velocity of mitral annulus; E, transmitral early left ventricular filling velocity; Ea, early diastolic velocity of mitral annulus.
Table 2.
Doppler tissue imaging in hypertrophic cardiomyopathy and controls
| Variable | HCM | Control | p Value |
| Lateral mitral annulus | |||
| Lateral Ea (cm/s) | 10 (3) | 18 (4) | <0.0001 |
| Lateral Aa (cm/s) | 11 (4) | 12 (3) | NS |
| Lateral Ea deceleration time (ms) | 117 (38) | 84 (19) | <0.0001 |
| Transmitral E/lateral Ea | 7.4 (3.2) | 4.3 (1.0) | <0.0001 |
| Septal mitral annulus | |||
| Septal Ea (cm/s) | 7 (2) | 12 (3) | <0.0001 |
| Septal Aa (cm/s) | 8 (3) | 10 (2) | <0.001 |
| Septal Ea deceleration time (ms) | 123 (30) | 101 (18) | <0.0001 |
| Transmitral E/septal Ea | 12.2 (9.6) | 6.3 (1.4) | <0.0001 |
Values are mean (SD).
Aa, late diastolic velocity of mitral annulus; DTI, Doppler tissue imaging; Ea, early diastolic velocity of mitral annulus; HCM, hypertrophic cardiomyopathy; transmitral E/lateral Ea, ratio of transmitral early left ventricular filling velocity to early diastolic velocity of lateral mitral annulus; transmitral E/septal Ea, ratio of transmitral early left ventricular filling velocity to early diastolic velocity of septal mitral annulus.
Doppler tissue imaging findings versus left ventricular hypertrophy, symptoms, and exercise capacity in hypertrophic cardiomyopathy
Although conventional Doppler indices did not correlate with the Wigle score, lateral Ea and septal Ea correlated with the Wigle score (lateral Ea: r = −0.35, p < 0.005; septal Ea: r = −0.31, p < 0.005).
None of the conventional Doppler indices correlated with PVo2. Lateral Ea was weakly correlated with PVo2 (lateral Ea: r = 0.28, p < 0.05). There was no correlation between septal Ea and PVo2. The transmitral E to lateral Ea ratio was inversely correlated with PVo2 (r = −0.42, p < 0.0001). This inverse correlation was seen in patients with and without left ventricular outflow tract obstruction (r = −0.40, p < 0.05; and r = −0.40, p < 0.005, respectively).
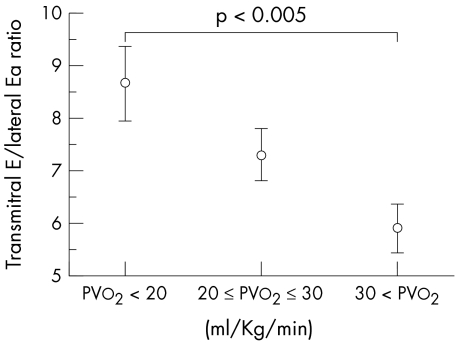
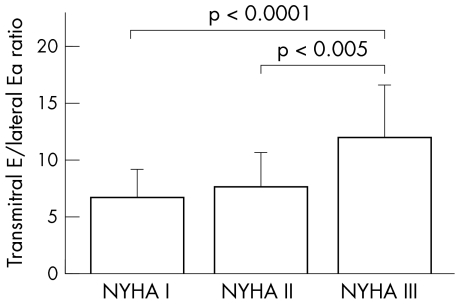
Three groups of the patients were distinguished: those with PVo2 (ml/kg/min) > 30, those with PVo2 between 20 and 30, and those with PVo2 < 20. The patients with PVo2 < 20 had a significantly higher transmitral E to lateral Ea ratio than those with PVo2 > 30 (8.6 (3.7) v 5.9 (2.2); p < 0.005) (fig 2). Patients in NYHA class III had a higher transmitral E to lateral Ea ratio (12.0 (4.6)) than those in NYHA class II (7.6 (3.1); p < 0.005) or class I (6.6 (2.6); p < 0.0001) (fig 3).
Figure 2.
Comparison of transmitral E to lateral Ea ratio in patients with hypertrophic cardiomyopathy with different capacity for exercise. Three groups of the patients were distinguished: those with peak oxygen consumption (PVo2; ml/kg/min) > 30, those with PVo2 between 20 and 30, and those with PVo2 < 20. The patients with PVo2 < 20 had significantly higher transmitral E to lateral Ea ratio than those with PVo2 > 30. NYHA, New York Heart Association; transmitral E/lateral Ea, ratio of transmitral early left ventricular filling velocity to early diastolic velocity of the lateral mitral annulus.
Figure 3.
Comparison of transmitral E to lateral Ea ratio in patients with hypertrophic cardiomyopathy in different New York Heart Association (NYHA) functional classes. Patients in NYHA class III had higher transmitral E to lateral Ea ratio than those in NYHA class II and class I. Transmitral E/lateral Ea, ratio of transmitral early left ventricular filling velocity to early diastolic velocity of the lateral mitral annulus.
DISCUSSION
This study shows that early diastolic mitral annular velocities measured using Doppler tissue imaging are reduced in patients with hypertrophic cardiomyopathy. Unlike conventional Doppler indices alone, the transmitral E to lateral Ea ratio correlated with NYHA functional class and exercise capacity.
Conventional echocardiographic assessment of diastolic function in hypertrophic cardiomyopathy
Many invasive pressure studies have shown that patients with hypertrophic cardiomyopathy have a spectrum of diastolic abnormalities, including increased mean left atrial and left ventricular end diastolic pressures, prolonged time constant of relaxation, and increased effective chamber and myocardial stiffness.3, 5 Most clinical studies have used non-invasive methods to assess diastolic function—in particular, pulsed wave Doppler interrogation of transmitral left ventricular filling velocities. Some previous studies showed that characteristic findings based on mitral inflow patterns are lower E wave velocity, prolonged E wave deceleration time, higher A wave velocity, and an E/A ratio < 1.0, which are clearly differentiated from normal inflow patterns in normal subjects.6, 25, 26 However, the overlap is considerable.6 Recent studies showed that most of the patients had a normal (or pseudonormalised) mitral inflow pattern.27–29
An invasive study produced some important results11: the mitral inflow velocity curve variables and the mean left atrial pressure were not related in patients with hypertrophic cardiomyopathy. Because of the complexity of the multiple and interrelated factors that determine left ventricular diastolic filling, the mitral inflow velocity curve is strongly influenced by factors independent of diastolic properties, such as loading conditions and age.13, 14
The limitations of conventional Doppler indices in patients with hypertrophic cardiomyopathy are illustrated in this study by the fact that, with the exception of E deceleration time and isovolumic relaxation time, the mean values for transmitral filling velocities were similar to those seen in controls. Although pulmonary venous Doppler velocities have been used to detect pseudonormalisation in patients with congestive heart failure, recent studies have shown that in patients with hypertrophic cardiomyopathy, pulmonary systolic and diastolic velocities bear little relation to left ventricular diastolic pressures.12, 30, 31 Our study confirms that many patient with hypertrophic cardiomyopathy have preservation of the pulmonary systolic wave with reduction in the diastolic wave velocity.
Diastolic mitral annular velocities and clinical features in hypertrophic cardiomyopathy
Previous studies in hypertrophic cardiomyopathy have shown that the transmural diastolic velocity gradient in the left ventricular posterior wall is reduced.32, 33 Our study confirms that early diastolic mitral annular velocities are reduced in patients with hypertrophic cardiomyopathy. Lateral Ea and septal Ea correlated with the Wigle score. Nevertheless, the correlation coefficients were modest, suggesting that diastolic longitudinal dysfunction is also determined by factors independent of severe hypertrophy, such as myocardial fibrosis, myocyte disarray, and diastolic ventricular interaction.
Our study also shows that, unlike conventional Doppler indices alone, the transmitral E to lateral Ea ratio correlates with NYHA functional class. It also shows that this ratio correlates with PVo2 in patients with hypertrophic cardiomyopathy with and without left ventricular outflow tract obstruction. As it has been shown recently that left ventricular filling pressures in patients with hypertrophic cardiomyopathy correlate with the transmitral E to lateral Ea ratio, these observations support the long held belief that dyspnoea and exercise intolerance in patients with hypertrophic cardiomyopathy are related largely to raised left atrial pressures.3, 5, 12
On the other hand, previous studies have emphasised that raised left atrial pressure is not a major determinant of exercise capacity in hypertrophic cardiomyopathy.34 Lele and colleagues suggest that stroke volume augmentation—which is determined by exercise diastolic filling characteristics—is the major determinant of peak exercise capacity in affected individuals.35 However, there is a limitation in that the study patients were not classified according to the presence or absence of left ventricular outflow obstruction.
Chikamori and colleagues assessed the relation of exercise capacity to indices of resting systolic and diastolic function using a nuclear technique in patients with and without a left ventricular outflow gradient.36 Their study suggests that there are different mechanisms of exercise limitation in hypertrophic cardiomyopathy: in patients with a left ventricular outflow gradient at rest, the main determinants of exercise limitation were impaired left ventricular and left atrial systolic performance; in those without a gradient, however, diastolic function at rest was a more important factor in the limitation of exercise performance.
A recent study showed that maximum oxygen consumption correlated with the left atrial fractional shortening, which is closely related to left ventricular end diastolic pressure at rest.37 These results support the role of left ventricular diastolic dysfunction at rest in limiting the exercise capacity of patients with hypertrophic cardiomyopathy.
It still seems to be controversial whether or not left ventricular diastolic pressure and function at rest are major determinants of exercise capacity in hypertrophic cardiomyopathy, and the true mechanism of exercise limitation remains unclear. In our present study, the correlation between the transmitral E to lateral Ea ratio and PVo2 was relatively modest, suggesting that other factors such as a reduced stroke volume response, ventilation/perfusion mismatch, and abnormal peripheral oxygen utilisation also influence exercise limitation.24, 34–36 Further studies are needed to determine the exact mechanisms of impaired exercise tolerance.
Conclusions
Early diastolic mitral annular velocities are reduced in patients with hypertrophic cardiomyopathy and are related to the magnitude of left ventricular hypertrophy. The transmitral E to lateral Ea ratio correlates with NYHA functional class and exercise capacity.
Acknowledgments
MSV was supported by a grant from the British Heart Foundation. We thank Gillian C Smith, Brian Mist, Annie O'Donoghue, and Shaughan Dickie for their assistance with this report. This study was presented in part at the 16th Annual Congress of the American College of Cardiology, New Orleans, March 1999.
Abbreviations
A, transmitral late left ventricular filling velocity
Aa, late diastolic velocity of mitral annulus
E, transmitral early left ventricular filling velocity
Ea, early diastolic velocity of mitral annulus
REFERENCES
- 1.Sanderson JE, Traill TA, St John Sutton MG, et al. Left ventricular relaxation and filling in hypertrophic cardiomyopathy. An echocardiographic study. Br Heart J 1978;40:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.St John Sutton MG, Tajik AJ, Gibson DG, et al. Echocardiographic assessment of left ventricular filling and septal and posterior wall dynamics in idiopathic hypertrophic subaortic stenosis. Circulation 1978;57:512–20. [DOI] [PubMed] [Google Scholar]
- 3.Hirota Y, Furubayashi K, Kaku K, et al. Hypertrophic nonobstructive cardiomyopathy: a precise assessment of hemodynamic characteristics and clinical implications. Am J Cardiol 1982;50:990–7. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Frederick TM, Bacharach SL, et al. Atrial systole and left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. Am J Cardiol 1983;51:1386–91. [DOI] [PubMed] [Google Scholar]
- 5.Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy: the importance of the site and the extent of hypertrophy: a review. Prog Cardiovasc Dis 1985;28:1–83. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Spirito P, Green KJ, et al. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 1987;10:733–42. [DOI] [PubMed] [Google Scholar]
- 7.Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990;15:808–13. [DOI] [PubMed] [Google Scholar]
- 8.Chikamori T, Dickie S, Poloniecki JD, et al. Prognostic significance of radionuclide-assessed diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1990;65:478–82. [DOI] [PubMed] [Google Scholar]
- 9.Nihoyannopoulos P, Karatasakis G, Frenneaux M, et al. Diastolic function in hypertrophic cardiomyopathy: relation to exercise capacity. J Am Coll Cardiol 1992;19:536–40. [DOI] [PubMed] [Google Scholar]
- 10.Pak PH, Maughan WL, Baughman KL, et al. Marked discordance between dynamics and passive diastolic pressure–volume relations in idiopathic hypertrophic cardiomyopathy. Circulation 1996;94:52–60. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, Appleton CP, Redfield MM, et al. Noninvasive Doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol 1996;28:1226–33. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999;99:254–61. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K, Redfield MM, Nishimura RA. Analysis of left ventricular diastolic function. Heart 1996;75(suppl 2):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988;12:426–40. [DOI] [PubMed] [Google Scholar]
- 15.Isaaz K, Thompson A, Ethevenot G, et al. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol 1989;64:66–75. [DOI] [PubMed] [Google Scholar]
- 16.Isaaz K, Munoz del Romeral L, Lee E, et al. Quantitation of the motion of the cardiac base in normal subjects by Doppler echocardiography. J Am Soc Echocardiogr 1993;6:166–76. [DOI] [PubMed] [Google Scholar]
- 17.Sohn D-W, Chai I-H, Lee D-J, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–80. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and filling pressures. J Am Coll Cardiol 1997;30:1527–33. [DOI] [PubMed] [Google Scholar]
- 19.Garcia MJ, Rodriguez L, Ares M, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy: assessment of left ventricular diastolic velocities in longitudinal axis by Doppler tissue imaging. J Am Coll Cardiol 1996;27:108–14. [DOI] [PubMed] [Google Scholar]
- 20.Report of the 1995 World Health Organization/International society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation 1996;93:841–2. [DOI] [PubMed] [Google Scholar]
- 21.Maron BJ, Gottdiener JS, Epstein SE. Patterns and significance of distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy. A wide angle, two-dimensional echocardiographic study of 125 patients. Am J Cardiol 1981;48:418–28. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro LM, McKenna WJ. Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: a two-dimensional echocardiographic study. J Am Coll Cardiol 1983;2:437–44. [DOI] [PubMed] [Google Scholar]
- 23.Mulvagh S, Quinones MA, Kleiman NS, et al. Estimation of left ventricular end-diastolic pressure from Doppler transmitral flow velocity in cardiac patients independent of systolic performance. J Am Coll Cardiol 1992;20:112–19. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Elliott PM, Sharma S, et al. Cardiopulmonary response to exercise in patients with hypertrophic cardiomyopathy. Heart 1998;80:60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gidding SS, Snider AR, Rocchini AP, et al. Left ventricular diastolic filling in children with hypertrophic cardiomyopathy: assessment with pulsed Doppler echocardiography. J Am Coll Cardiol 1986;8:310–16. [DOI] [PubMed] [Google Scholar]
- 26.Bonow RO, Dilsizian V, Rosing DR, et al. Verapamil-induced improvement in left ventricular diastolic filling and increased exercise tolerance in patients with hypertrophic cardiomyopathy: short- and long-term effects. Circulation 1985;72:853–64. [DOI] [PubMed] [Google Scholar]
- 27.Hada Y, Ito N, Asakawa M, et al. Left ventricular wall motion dynamics of asymmetric septal hypertrophy: assessment by intramyocardial pulsed Doppler echocardiography. J Cardiol 1998;31:351–60. [PubMed] [Google Scholar]
- 28.Briguori C, Betocchi S, Losi MA, et al. Noninvasive evaluation of left ventricular diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1998;81:180–7. [DOI] [PubMed] [Google Scholar]
- 29.Severino S, Caso P, Galderisi M, et al. Use of pulsed Doppler tissue imaging to assess regional left ventricular diastolic dysfunction in hypertrophic cardiomyopathy. Am J Cardiol 1998;82:1394–8. [DOI] [PubMed] [Google Scholar]
- 30.Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures. J Am Coll Cardiol 1993;21:1687–96. [DOI] [PubMed] [Google Scholar]
- 31.Appleton CP, Galloway JM, Gonzalez MS, et al. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol 1993;22:1972–82. [DOI] [PubMed] [Google Scholar]
- 32.Palka P, Lange A, Fleming AD, et al. Differences in myocardial velocity gradient measured throughout the cardiac cycle in patients with hypertrophic cardiomyopathy, athletes and patients with left ventricular hypertrophy due to hypertension. J Am Coll Cardiol 1997;30:760–8. [DOI] [PubMed] [Google Scholar]
- 33.Oki T, Mishiro Y, Yamada H, et al. Detection of left ventricular regional relaxation abnormalities and asynchrony in patients with hypertrophic cardiomyopathy with use of tissue Doppler imaging. Am Heart J 2000;139:497–502. [DOI] [PubMed] [Google Scholar]
- 34.Frenneaux MP, Porter A, Caforio ALP, et al. Determinants of exercise capacity in hypertrophic cardiomyopathy. J Am Coll Cardiol 1989;13:1521–6. [DOI] [PubMed] [Google Scholar]
- 35.Lele SS, Thomson HL, Seo H, et al. Exercise capacity in hypertrophic cardiomyopathy. Role of stroke volume limitation, heart rate and diastolic filling characteristics. Circulation 1995;92:2886–94. [DOI] [PubMed] [Google Scholar]
- 36.Chikamori T, Counihan PJ, Doi YL, et al. Mechanism of exercise limitation in hypertrophic cardiomyopathy. J Am Coll Cardiol 1992;19:507–12. [DOI] [PubMed] [Google Scholar]
- 37.Briguori C, Betocchi S, Romano M, et al. Exercise capacity in hypertrophic cardiomyopathy depends on left ventricular diastolic function. Am J Cardiol 1999;84:309–15. [DOI] [PubMed] [Google Scholar]