Abstract
Background: Angiotensin 1 converting enzyme (ACE) inhibitors reduce morbidity and mortality after coronary artery bypass graft surgery (CABG). This benefit may result from an anti-inflammatory action.
Objective: To examine the effect of ACE inhibition on interleukin 6 (IL-6) concentrations after CABG.
Patients and methods: 161 patients undergoing elective first time CABG were recruited, of whom 41 (25%) were receiving ACE inhibitor treatment; 21 patients with confounding postoperative complications were excluded. After these exclusions there were 33 patients (24%) on ACE inhibitor treatment. Plasma IL-6 was measured preoperatively and again six hours after CABG.
Results: Baseline IL-6 concentrations (geometric mean (SEM)) were non-significantly lower among the patients receiving ACE inhibitors (3.7 (0.1) v 4.3 (0.1) pg/ml, p = 0.12). Overall, post-CABG IL-6 concentrations increased significantly (mean rise 177 (12) pg/ml, p < 0.0005). This response was blunted among ACE inhibitor treated patients. Median increases in IL-6 concentrations were 117 v 193 pg/ml, for treated v non-treated patients, respectively (Kruskal–Wallis, p = 0.02), with peak postoperative IL-6 concentrations lower among the subjects receiving ACE inhibitors than in untreated subjects (142 (19) v 196 (13) pg/ml, p = 0.02). The effect of ACE inhibitors remained significant after multivariate analysis (p = 0.018).
Conclusions: ACE inhibitor treatment is associated with a reduction in IL-6 response to CABG. The data suggest that this class of drug may have a direct anti-inflammatory effect, which could explain some of its clinical benefit.
Keywords: angiotensin converting enzyme, inflammation, interleukin 6, coronary artery bypass graft
Treatment with angiotensin 1 converting enzyme (ACE) inhibitors is associated with substantial reductions in cardiovascular mortality and morbidity among patients with impaired myocardial function.1, 2 The HOPE (heart outcomes prevention evaluation) study3 has extended our knowledge of the benefits of ACE inhibition in people who are at coronary risk but have normal left ventricular function, with reductions in mortality, myocardial infarction, cardiac arrest, and heart failure. A recent meta-analysis has also confirmed that a substantial reduction in sudden cardiac deaths occurs in patients treated with an ACE inhibitor early after myocardial infarction.4 These beneficial effects are inadequately explained by the simple hypotensive and natriuretic effects of systemic inhibition of the renin–angiotensin system.3, 5 It is now thought likely that an anti-inflammatory action is involved.6
Local (myocardial) or systemic inflammatory processes could play key roles in the progression of heart failure,7–9 while local (coronary plaque) or systemic inflammation could mediate atheromatous plaque growth10 and initiate unstable plaque events.11 It is thought that the pro-inflammatory cytokine interleukin 6 (IL-6) plays a central role in this. Thus IL-6 concentrations are raised in patients with heart failure12 and indeed may play a causative role in driving heart failure by stimulating cardiomyocyte apoptosis,8 while IL-6 induced endothelial activation13, 14 together with vascular smooth muscle cell proliferation15 and migration16 may underlie the atherogenic process.17
Raised systemic concentrations of IL-6 are thus associated with both the development and the progression of heart failure.12, 18, 19 They are also related to the development of coronary vascular disease,20, 21 as well as the transition to plaque instability.11 Raised concentrations of IL-6 are also correlated with mortality in elderly people.22, 23 Atherosclerosis is itself a chronic low grade inflammatory condition characterised by activation of the acute phase response,24 and thus local plaque IL-6 expression may exert important effects. Both vascular smooth muscle cells and inflammatory cells such as macrophages are key components of the atherosclerotic plaque,25, 26 in which a high tissue ACE expression increases further upon inflammatory stimulation.27 Concomitant increases in plaque angiotensin II expression28 may drive IL-6 expression. IL-6 is co-localised with ACE within atherosclerotic plaques,29, 30 suggesting a possible local role of inflammation in the initiation and progression of atherosclerosis.17 Plaque ACE is thus considered an important therapeutic target in its own right.27 It has thus been postulated that the therapeutic benefits of ACE inhibition may be partly mediated through an anti-inflammatory action, and specifically through reductions in local or systemic IL-6 expression.31
The acute phase response following coronary artery bypass graft surgery (CABG) is associated with the induction and release of cytokines, including IL-6,32–34 and affords investigators a useful opportunity to study the impact of various factors on the acute phase response. However, to date the impact of ACE inhibitors on post-CABG IL-6 response has not been investigated. We have thus used this model to perform an observational study exploring the hypothesis that ACE inhibition is associated with a reduction in the IL-6 response to acute inflammatory stimuli.
METHODS
Subjects were drawn from the coronary artery surgery inflammation study (CASIS). Briefly, all patients undergoing elective first time CABG at the Middlesex Hospital, London, UK, between October 1999 and September 2000 were invited to participate. The study had hospital ethics committee approval, and all patients gave written informed consent.
Subjects undergoing additional surgical procedures (such as valvar surgery or aneurysmectomy) were excluded.
All patients were taking aspirin 75 mg/day, and ceased taking this 10 days before surgery. Other drugs were withheld on the morning of surgery. ACE inhibitor treatment was given as usual the night before surgery. No patient had clinical evidence of active inflammatory or immunomodulatory disease (such as acute coronary syndromes, malignancy, intercurrent infection, renal failure, or autoimmune disease) at recruitment, nor were any receiving anti-inflammatory agents. Similarly, before analysis all data were excluded from those subjects who experienced significant postoperative complications, even if these were only apparent after the six hour sample time point, as IL-6 rises within hours of an inflammatory stimulus. Study exclusion criteria thus included infections requiring antibiotic treatment, prolonged respiratory support, circulatory support, or renal failure requiring haemofiltration.
Surgical procedure
CABG was performed through a midline sternotomy by one of four consultant surgical staff, with subsequent hypothermic cardiopulmonary bypass employing right atrial and ascending aortic cannulation. Myocardial protection was maintained by intermittent cold cross clamp fibrillation, and heparin anticoagulation was reversed postoperatively by protamine sulfate.
A 4.5 ml citrated blood sample was drawn before surgery (with the patient supine and resting) and again six hours after cardiopulmonary bypass. Venous blood was immediately centrifuged (at 3500 g for 10 minutes), and the plasma was separated and stored at −20°C until analysis. IL-6 concentrations were measured by enzyme linked immunosorbent assay (R&D Systems, Abingdon, UK) by staff blind to all subject data. Interassay and intra-assay coefficients of variation were 5% and 3%, respectively, and assay sensitivity was < 0.70 pg/ml).
Statistical analysis
All data were analysed using SPSS for Windows version 9 (SPSS Inc, Chicago, Illinois, USA). We analysed raw data when they were normally distributed. However, the IL-6 values were skewed, so they were normalised by log transformation (the data shown represent geometric means and standard deviations). The effect of ACE inhibitor treatment on IL-6 was assessed by analysis of variance and Student's t test for unpaired data. One way analysis of covariance was performed using age, sex, smoking, diabetic status, left ventricular ejection fraction, statin treatment, duration of operation, and cardiopulmonary bypass and aortic cross clamp times as covariates. Following backwards stepwise linear regression modelling, only variables that had a significant effect on IL-6 concentrations were included in the final model. Pearson's correlation coefficient was used to test the relation between IL-6 concentrations and baseline clinical and surgical variables. The study was powered to detect a 10% difference in post-CABG IL-6 with 80% power at p < 0.05.
RESULTS
Of 269 elective CABG cases, 172 who fulfilled the inclusion criteria were randomly approached (depending on investigator availability), and of these 161 chose to participate. At enrolment, 41 (25%) were receiving ACE inhibitor treatment: 11 (27%) were on lisinopril, 10 (24%) on ramipril, six (15%) on enalapril, six (15%) on perindopril, four (10%) on captopril, and four (10%) were taking other agents. In all, 111 subjects (67%) were receiving statin treatment. Twenty one patients experienced confounding postoperative complications and were thus excluded, according to protocol. This left a final study group of 140 subjects, including 33 (24%) receiving ACE inhibitors.
Clinical and operative characteristics (table 1) were largely independent of ACE inhibitor treatment, although body mass index was slightly lower. As anticipated, a past medical history of diabetes or hypertension was more common in ACE inhibitor treated patients. Similarly, a higher proportion of subjects receiving ACE inhibitors had a left ventricular ejection fraction below 50% (18/33 v 29/107, p < 0.01).
Table 1.
Patient baseline characteristics and operative details
| Variable | ACE inhibitor treatment (n=33) | No ACE inhibitor treatment (n=107) | p Value |
| Baseline characteristics | |||
| Men | 27 | 82 | |
| Women | 6 | 25 | |
| Age (years) | 64 (2) | 63 (1) | 0.73 |
| Body mass index (kg/m2) | 26.5 (0.4) | 28.4 (0.7) | 0.04 |
| Current smoker | 4 (13%) | 20 (19%) | 0.29 |
| Treated hypertension | 18 (55%) | 33 (31%) | 0.01 |
| Diabetes mellitus | 10 (30%) | 18 (17%) | 0.08 |
| Family history of coronary artery disease | 15 (46%) | 54 (51%) | 0.38 |
| Total cholesterol (mmol/l) | 4.8 (0.2) | 4.9 (0.1) | 0.74 |
| Low density lipoprotein (mmol/l) | 2.5 (0.2) | 2.6 (0.1) | 0.48 |
| High density lipoprotein (mmol/l) | 1.4 (0.1) | 1.3 (0.1) | 0.52 |
| Canadian Cardiovascular Society class | 2.6 (0.2) | 2.2 (0.1) | 0.11 |
| New York Heart Association class | 1.8 (0.1) | 1.8 (0.1) | 0.75 |
| Operative details | |||
| Mean number of grafts | 2.8 (0.1) | 2.8 (0.1) | 0.75 |
| Operation duration (minutes) | 190 (8) | 195 (3) | 0.49 |
| Cardiopulmonary bypass time (minutes) | 63 (3) | 66 (2) | 0.31 |
| Aortic cross clamp time (minutes) | 34 (3) | 32 (1) | 0.58 |
| Length of ventilation (hours) | 9.3 (0.9) | 10.3 (0.4) | 0.24 |
| Length of stay in intensive care unit (days) | 2.3 (0.5) | 2.3 (0.2) | 0.85 |
| Length of postoperative stay (days) | 7.0 (1.0) | 6.6 (0.4) | 0.64 |
Values are n (%) or mean (SEM).
ACE, angiotensin converting enzyme.
The correlation between baseline and post-CABG IL-6 values and the major clinical and surgical variables is shown in table 2. While both baseline and post-CABG IL-6 concentrations were lower in non-diabetic patients, men, and non-smokers, none of these factors was associated with a significant effect on IL-6 concentrations; however, post-CABG IL-6 concentrations were lower in patients receiving statin treatment (p = 0.029).
Table 2.
Correlation between IL-6 concentrations and clinical variables
| Variable | Preoperative IL-6 | Postoperative IL-6 |
| Age | r=0.05 (p=0.57) | r=−0.19 (p=0.02) |
| Body mass index | r=0.22 (p=0.04) | r=0.15 (p=0.15) |
| Ejection fraction | r=−0.12 (p=0.17) | r=−0.16 (p=0.08) |
| Aortic cross clamp time | – | r=0.08 (p=0.41) |
| Cardiopulmonary bypass time | – | r=0.14 (p=0.11) |
| Total duration of surgery | – | r=0.23 (p=0.01) |
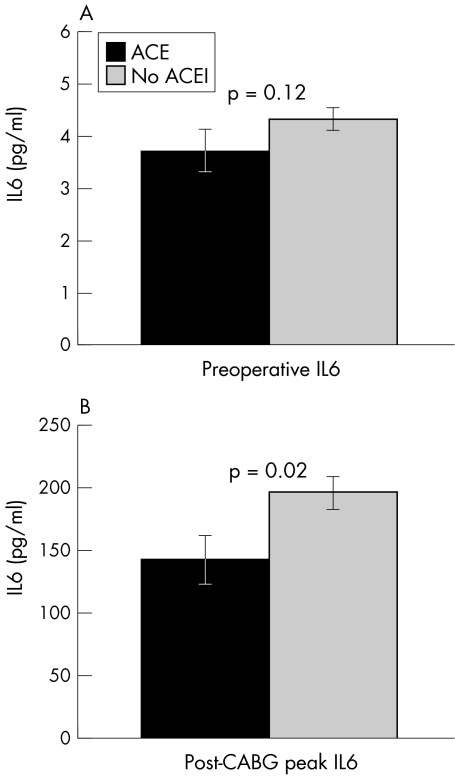
The effect of ACE inhibitor treatment on IL-6 concentrations is shown in fig 1. A trend towards lower preoperative IL-6 concentrations among patients receiving ACE inhibitors (mean (SD), 3.7 (0.1) v 4.3 (0.1) pg/ml, p = 0.12) became less strong after multivariate analysis. Among the group as a whole, surgery was associated with a significant increase in IL-6 concentrations (mean rise, 177 (12) pg/ml, p < 0.0005 by paired t test). However, this response was significantly blunted among ACE inhibitor treated patients: median increases in IL-6 concentrations were 117 pg/ml in treated patients and 193 pg/ml in non-treated patients (Kruskal–Wallis, p = 0.02), with peak postoperative IL-6 concentrations lower among the 33 patients receiving ACE inhibitors than in the 107 untreated subjects (142 (19) v 196 (13) pg/ml, p = 0.02). The effect of ACE inhibitor treatment on post-CABG IL-6 concentrations remained significant after multivariate analysis (analysis of covariance, p = 0.018).
Figure 1.
Effect of ACE inhibitor treatment on post-coronary artery bypass graft IL-6.
DISCUSSION
While the anti-inflammatory role of ACE inhibitors has been reported previously in vitro,35, 36 this study suggests for the first time that ACE inhibitor treatment is also associated with a reduction in IL-6 response to coronary artery bypass surgery. Although observational in nature and not derived from a randomised double blind control study, these data have important therapeutic implications in at least three fields.
Firstly, the pronounced inflammatory response to CABG is implicated in the pathogenesis of subsequent hyperdynamic circulatory instability, delayed myocardial recovery, and organ dysfunction.33, 37 More specifically, prolonged increases of circulating IL-6 are also associated with increased morbidity and mortality after cardiac operations.38 It has already been shown that ACE inhibition may improve outcome in patients undergoing CABG,39 although a mechanistic explanation for this observation remains elusive. Our data would support the suggestion that inherent anti-inflammatory properties of ACE inhibitor drugs may be responsible.35 However, as the study was designed only to include subjects with an uncomplicated postoperative course, it is unsurprising that there was no significant difference in the length of hospital stay between those patients who did or did not receive an ACE inhibitor.
Secondly, there is evidence of cross signalling between the renin–angiotensin system and IL-6, so the former may contribute to the pathogenesis of atherosclerosis. Angiotensin II stimulates IL-6 production by smooth muscle cells, an effect that can be inhibited by captopril and ramiprilat.36 Steady state mRNA for IL-6 can also be augmented after stimulation with angiotensin II, suggesting regulation of angiotensin induced IL-6 release at the pretranslational level.36 Moreover, angiotensin II can also activate the pro-inflammatory transcription factor nuclear factor κB, which is necessary for transcription of most cytokine genes, including IL-6.40 Thus it is likely that angiotensin can elicit an inflammatory response in human vascular smooth muscle cells by stimulating cytokine production and the activation of nuclear factor κB.36 Our data would support an anti-inflammatory role for ACE inhibition in reducing vascular event rates in those at risk.3
Finally, the dramatic benefit of ACE inhibition in patients with heart failure remains to be explained, although our data once again support a potential anti-inflammatory role in mediating those effects.41
It remains possible, of course, that it is not the ACE inhibition on its own that is associated with a reduced IL-6 response, but the condition that led to such treatment in the first place. However, we think that unlikely.42 If anything, the inflammatory response to surgery might be expected to be greater among patients with pre-existing cardiac dysfunction or diabetes.43 In any event, with the increasing indications for ACE inhibitor treatment (hypertension, diabetes,44 coronary artery disease,3 and cardiac dysfunction1, 2), there are ethical concerns about performing a randomised controlled trial of ACE inhibition in patients selected for coronary surgery.44
Clearly further studies are required, both in vitro, to investigate the effect of ACE inhibitor on IL-6 production using preparations of cells implicated in IL-6 synthesis (such as macrophages, fibroblasts, and endothelial cells), and in vivo, to explore the effect of ACE inhibition on the IL-6 response to other types and grades of inflammatory stimulus—for example, following acute coronary syndromes or coronary intervention. Any such studies need to be both randomised and double blind in design. Nonetheless, these data are supportive of an in vivo anti-inflammatory effect of ACE inhibition in humans.
Acknowledgments
We wish to thank all the patients who so kindly took part: Dr Andrew R Webb and his ICU/HDU staff, and the consultant surgical staff (Charles Pattison, Robin Walesby, Panny Kallis, and Shyam Kolvekar). DJB, HEM, AR, GDOL, and SEH are supported by the British Heart Foundation.
Abbreviations
ACE, angiotensin converting enzyme
CABG, coronary artery bypass graft
CASIS, coronary artery surgery inflammation study
HOPE, heart outcomes prevention evaluation study
IL-6, interleukin 6
REFERENCES
- 1.AIRE Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993;342:821–8. [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–91. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- 4.Domanski MJ, Exner DV, Borkowf CB, et al. Effect of angiotensin converting enzyme inhibition on sudden cardiac death in patients following acute myocardial infarction. A meta- analysis of randomised clinical trials. J Am Coll Cardiol 1993;33:598–604. [DOI] [PubMed] [Google Scholar]
- 5.Coats A. Interruption of the progression of heart failure: are ACE inhibitors the solution? Cardiology 1996;87(suppl 1):11–15. [DOI] [PubMed] [Google Scholar]
- 6.Curzen NK. Do ACE inhibitors modulate atherosclerosis? Eur Heart J 1997;10:1530. [DOI] [PubMed] [Google Scholar]
- 7.Shan K, Kurrelmeyer K, Seta Y, et al. The role of cytokines in disease progression in heart failure. Curr Opin Cardiol 1997;12:218–23. [DOI] [PubMed] [Google Scholar]
- 8.Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart Fail Rev 2001;6:95–103. [DOI] [PubMed] [Google Scholar]
- 9.Blum A, Miller H. Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med 2001;52:15–27. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. The interface of atherosclerosis and thrombosis: basic mechanisms. Vasc Med 1998;3:225–9. [DOI] [PubMed] [Google Scholar]
- 11.Liuzzo G, Baisucci LM, Gallimore JR, et al. Enhanced inflammatory response in patients with preinfarction unstable angina. J Am Coll Cardiol 1999;34:1696–703. [DOI] [PubMed] [Google Scholar]
- 12.Raymond RJ, Dehmer GJ, Theoharides TC, et al. Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction. Am Heart J 2001;141:435–8. [DOI] [PubMed] [Google Scholar]
- 13.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 1999;19:972–8. [DOI] [PubMed] [Google Scholar]
- 14.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997;6:315–25. [DOI] [PubMed] [Google Scholar]
- 15.Nabata T, Morimoto S, Koh E, et al. Interleukin-6 stimulates c-myc expression and proliferation of cultured vascular smooth muscle cells. Biochem Int 1990;20:445–53. [PubMed] [Google Scholar]
- 16.Zhu Y, Hojo Y, Ikeda U, et al. Interaction between monocytes and vascular smooth muscle cells enhances matrix metalloproteinase-1 production. J Cardiovasc Pharmacol 2000;36:152–61. [DOI] [PubMed] [Google Scholar]
- 17.Huber SA, Sakkinen P, Conze D, et al. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1999;19:2364–7. [DOI] [PubMed] [Google Scholar]
- 18.Munger MA, Johnson B, Amber IJ, et al. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1996;77:723–7. [DOI] [PubMed] [Google Scholar]
- 19.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998;31:391–8. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000;101:1767–72. [DOI] [PubMed] [Google Scholar]
- 22.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 1999;106:506–12. [DOI] [PubMed] [Google Scholar]
- 23.Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation 2001;103:947–53. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seino Y, Ikeda U, Takahashi M, et al. Expression of monocyte chemoattractant protein-1 in vascular tissue. Cytokine 1995;7:575–9. [DOI] [PubMed] [Google Scholar]
- 26.Galis ZS, Muszynski M, Sukhova GK, et al. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann NY Acad Sci 1995;748:501–7. [DOI] [PubMed] [Google Scholar]
- 27.Diet F, Pratt RE, Berry GJ, et al. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation 1996;94:2756–67. [DOI] [PubMed] [Google Scholar]
- 28.Schieffer B, Schieffer E, Hilfiker-Kleiner D, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation 2000;101:1372–8. [DOI] [PubMed] [Google Scholar]
- 29.Seino Y, Ikeda U, Ikeda M, et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine 1994;6:87–91. [DOI] [PubMed] [Google Scholar]
- 30.Rus HG, Vlaicu R, Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 1996;127:263–71. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Presa MA, Bustos C, Ortego M, et al. ACE inhibitor quinapril reduces the arterial expression of NF-kappaB-dependent proinflammatory factors but not of collagen I in a rabbit model of atherosclerosis. Am J Pathol 1998;153:1825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitten CW, Hill GE, Ivy R, et al. Does the duration of cardiopulmonary bypass or aortic cross-clamp, in the absence of blood and/or blood product administration, influence the IL-6 response to cardiac surgery? Anesth Analg 1998;86:28–33. [DOI] [PubMed] [Google Scholar]
- 33.Liebold A, Langhammer T, Brunger F, et al. Cardiac interleukin-6 release and myocardial recovery after aortic crossclamping. Crystalloid versus blood cardioplegia. J Cardiovasc Surg 1999;40:633–6. [PubMed] [Google Scholar]
- 34.Corbi P, Rahmati M, Delwail A, et al. Circulating soluble gp130, soluble IL-6R, and IL-6 in patients undergoing cardiac surgery, with or without extracorporeal circulation. Eur J Cardiothorac Surg 2000;18:98–103. [DOI] [PubMed] [Google Scholar]
- 35.Peeters AC, Netea MG, Kullberg BJ, et al. The effect of renin-angiotensin system inhibitors on pro- and anti-inflammatory cytokine production. Immunology 1998;94:376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kranzhofer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1999;19:1623–9. [DOI] [PubMed] [Google Scholar]
- 37.Cremer J, Martin M, Redl H, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996;61:1714–20. [DOI] [PubMed] [Google Scholar]
- 38.Roytblat L, Talmor D, Rachinsky M, et al. Ketamine attenuates the interleukin-6 response after cardiopulmonary bypass. Anesth Analg 1998;87:266–71. [DOI] [PubMed] [Google Scholar]
- 39.Oosterga M, Voors AA, Pinto YM, et al. Effects of quinapril on clinical outcome after coronary artery bypass grafting (The QUO VADIS Study). QUinapril on Vascular Ace and Determinants of Ischemia. Am J Cardiol 2001;87:542–6. [DOI] [PubMed] [Google Scholar]
- 40.Han Y, Runge MS, Brasier AR. Angiotensin II induces interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kappa B transcription factors. Circ Res 1999;84:695–703. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez-Presa M, Bustos C, Ortego M, et al. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 1997;95:1532–41. [DOI] [PubMed] [Google Scholar]
- 42.Matata BM, Galinanes M. Cardiopulmonary bypass exacerbates oxidative stress but does not increase proinflammatory cytokine release in patients with diabetes compared with patients without diabetes: regulatory effects of exogenous nitric oxide. J Thorac Cardiovasc Surg 2000;120:1–11. [DOI] [PubMed] [Google Scholar]
- 43.Deng MC, Dasch B, Erren M, et al. Impact of left ventricular dysfunction on cytokines, hemodynamics, and outcome in bypass grafting. Ann Thorac Surg 1996;62:184–90. [DOI] [PubMed] [Google Scholar]
- 44.O'Keefe JH, Wetzel M, Moe RR, et al. Should an angiotensin-converting enzyme inhibitor be standard therapy for patients with atherosclerotic disease? J Am Coll Cardiol 2001;37:1–8. [DOI] [PubMed] [Google Scholar]