Abstract
Background: There is evidence that adrenomedullin has autocrine or paracrine activities that oppose cardiac remodelling. However, it remains unclear whether it exerts those local functions in heart failure patients.
Objective: To investigate the relation between plasma and pericardial fluid concentrations of adrenomedullin and left ventricular haemodynamic variables.
Design: Samples of plasma and pericardial fluid were obtained from 50 patients undergoing cardiac surgery. They were classified into two groups: group N (n = 27) with a left ventricular end diastolic volume index (LVEDVI) ≤ 90 ml/m2; and group R (n = 23) with LVEDVI > 90 ml/m2. Plasma and pericardial fluid concentrations of total adrenomedullin (tAM) and mature adrenomedullin (mAM) were measured and related to the preoperative haemodynamic variables.
Results: Pericardial fluid concentrations of mAM were much higher than the plasma concentration in both group N and group R (mean (SEM), 10.6 (1.7) v 3.3 (0.2) fmol/ml, p = 0.0001; and 21.2 (2.8) v 3.9 (0.3) fmol/ml, p < 0.0001, respectively). The ratio mAM/tAM in pericardial fluid was significantly higher than in plasma (0.56 (0.02) v 0.28 (0.02), p < 0.0001). Pericardial fluid concentrations of mAM, but not plasma concentrations, were significantly correlated with LVEDVI, left ventricular end systolic volume index, left ventricular ejection fraction, and left ventricular mass index (r = 0.60, 0.63, −0.54, and 0.47, respectively).
Conclusions: Raised pericardial fluid concentrations of mAM may reflect the actions of adrenomedullin as a local mediator against cardiac remodelling in patients with left ventricular dysfunction.
Keywords: adrenomedullin, cardiac remodelling, heart failure, pericardial fluid
Adrenomedullin, originally isolated from human phaeochromocytoma, is a potent vasodilating peptide that also has natriuretic and diuretic actions.1, 2 Human adrenomedullin consists of 52 amino acid residues that have a ring structure formed by an intramolecular disulphide bridge and a tyrosine amide at its C-terminal residue,1 both of which are essential for hypotensive and other biological activities.3 In the biosynthesis of adrenomedullin, an intermediate form—adrenomedullin(1-52)-glycine-COOH (iAM)—is processed from proadrenomedullin and subsequently converted to a biologically active mature form, adrenomedullin(1-52)-NH2 (mAM), by enzymatic amidation.4 Therefore, although total immunoreactive adrenomedullin (tAM) represents not only mAM but also iAM, only mAM exerts significant biological activities.5 Recently, it was reported that plasma concentrations of both mAM and iAM were progressively increased in proportion to the severity of heart failure.6, 7
However, there is also increasing evidence of autocrine or paracrine modes of action of adrenomedullin in various organs8 including the heart.9–14 Recent reports suggested that adrenomedullin secreted from cardiac myocytes and fibroblasts may exert its biological effects to counteract pressure and volume overload of the heart in rat models.10–14 However, there have been no clinical studies on how adrenomedullin of cardiac origin exerts its local pathophysiological functions in the process of cardiac remodelling in failing hearts.
Our aims in the present study were to measure plasma and pericardial fluid concentrations of tAM and mAM in patients undergoing cardiac surgery, and to investigate their relations with haemodynamic variables in cardiac remodelling.
METHODS
Patients
We enrolled 50 consecutive patients (34 men and 16 women) who underwent cardiac surgery at Kyoto University Hospital and Takeda Hospital. There were no exclusion criteria except failure to obtain informed consent. The mean age of the patients was 65.8 years (range 46–87 years). All were clinically evaluated before operation by echocardiography and cardiac catheterisation. They underwent M mode and cross sectional echocardiography and single or biplane left ventriculography according to standard techniques. Left ventricular mass was calculated from the Penn convention according to the equation of Devereux and Reichek,15 and indexed to body surface area, forming the left ventricular mass index (LVMI). Left ventricular end diastolic and end systolic volume indices (LVEDVI and LVESVI) and left ventricular ejection fraction (LVEF) were calculated from left ventricular cineangiography in the right anterior oblique projection.16 Left ventricular end diastolic pressure (LVEDP) was also measured. The patients were classified into two groups depending on their LVEDVI values. Group N (the “normal” group) consisted of 27 patients with an LVEDVI of ≤ 90 ml/m2; group R (the “remodelling” group) consisted of 23 patients with an LVEDVI of > 90 ml/m2.
All the patients gave their written informed consent. The study protocol was approved by the ethics committees on human research of both Kyoto University Hospital and Takeda Hospital.
Sampling of plasma and pericardial fluid
Blood and pericardial fluid samples were obtained during operation from all the patients. With the exception of β blockers, all oral drug treatment was all discontinued 12–18 hours before surgery. Immediately after incision of the pericardium, undiluted pericardial fluid was collected before heparinisation, except in a few patients with unstable angina who had a continuous heparin infusion. At the same time, blood was drawn from the radial arterial line. These samples were immediately transferred into chilled sterile tubes containing disodium EDTA (1 mg/ml) and aprotinin (500 U/ml). They were centrifuged immediately at 2500 ×g for 15 minutes at 4°C. The clarified plasma and pericardial fluid samples were frozen and stored at −80°C, and thawed just before immunoradiometric assay.
Measurement of tAM and mAM in plasma and pericardial fluid
The measurement of tAM and mAM in plasma and pericardial fluid was performed by immunoradiometric assay using a specific kit for each form (adrenomedullin RIA Shionogi, adrenomedullin mature RIA Shionogi; Cosmic Corporation, Tokyo, Japan). These kits were designed to follow the methods developed by Ohta and colleagues.7, 17 These investigators reported that no cross reactivity was observed with partial fragments of adrenomedullin or other peptides similar to adrenomedullin in either assay, and that iAM was not detected in the mAM assay.
Statistical analyses
Numerical data are expressed as mean (SEM). Proportion analysis between groups N and R was made by a χ2 test or Fisher's exact test. Comparisons of variables between the two groups were made by Student's unpaired t test or the Mann–Whitney U test. Comparisons of concentrations among each group were performed by Wilcoxon's signed rank test. Multiplicity for statistical tests was adjusted by Bonferroni's method. Student's paired t test and Spearman's correlation coefficients were used in assessing the ratio of the mAM concentration to the tAM concentration. Spearman's correlation coefficients were also used to evaluate the relations between adrenomedullin concentrations and preoperative haemodynamic variables. A probability value of p < 0.05 was considered significant.
RESULTS
Patient characteristics
Table 1 shows clinical profiles of the study patients in groups N and R. No differences were observed in age or sex. There were no significant differences between the two groups with regard to the proportions of patients who had hypertension, renal failure, positive serum concentrations of C reactive protein, diabetes mellitus, unstable angina, or the acute phase of myocardial infarction. Group R contained significantly more patients with a history of myocardial infarction than group N. There were no differences between the groups in the preoperative use of β blockers or angiotensin converting enzyme (ACE) inhibitors, both of which have actions against cardiac remodelling.
Table 1.
Clinical characteristics of the patients in group N (no remodelling) and group R (remodelling)
| Characteristic | Group N (n=27) | Group R (n=23) | p Value |
| Age (years) | 65 (2) | 66 (2) | 0.67 |
| Sex (male/female) | 20/7 | 14/9 | 0.32 |
| NYHA functional class | 0.0063 | ||
| I | 11 | 2 | |
| II | 9 | 8 | |
| III | 6 | 10 | |
| IV | 1 | 3 | |
| Diagnosis | |||
| Coronary artery disease | 17 | 14 | 0.88 |
| Valvar heart disease | 7 | 12 | 0.057 |
| Thoracic aortic disease | 4 | 0 | 0.11 |
| Other | 1 | 0 | >0.9999 |
| Comorbidities | |||
| Hypertension | 14 | 15 | 0.34 |
| Renal failure | 6 | 6 | 0.19 |
| Positive CRP | 4 | 1 | 0.36 |
| Diabetes mellitus | 10 | 8 | >0.9999 |
| Unstable angina | 0 | 3 | 0.090 |
| Acute myocardial infarction | 1 | 2 | 0.59 |
| Old myocardial infarction | 3 | 9 | 0.021 |
| Haemodynamic variables | |||
| Heart rate (beats/min) | 72 (3) | 78 (3) | 0.10 |
| MAP (mm Hg) | 89 (2) | 89 (3) | 0.85 |
| LVEDVI (ml/m2) | 65 (3) | 121 (5) | <0.0001 |
| LVESVI (ml/m2) | 24 (3) | 71 (5) | <0.0001 |
| LVEF (%) | 66 (3) | 42 (3) | <0.0001 |
| LVMI (g/m2) | 141 (9) | 193 (14) | 0.0017 |
| LVEDP (mm Hg) | 12 (1) | 15 (2) | 0.097 |
| Preoperative drug use | |||
| Heparin infusion | 2 | 3 | 0.65 |
| β Blockers | 4 | 4 | 0.80 |
| ACE inhibitors | 12 | 11 | 0.81 |
Values are n or mean (SEM).
ACE, angiotensin converting enzyme; CRP, C reactive protein; LVEDP, left ventricular end diastolic pressure; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end systolic volume index; LVMI, left ventricular mass index; MAP, mean aortic pressure; NYHA, New York Heart Association.
Plasma and pericardial fluid concentrations of tAM
Pericardial fluid concentrations of tAM were higher than the plasma concentrations in group R (38.2 (4.5) v 18.7 (2.3) fmol/ml, p = 0.0001), while there were no significant differences between pericardial fluid and plasma levels in group N (18.6 (2.8) v 12.7 (1.3) fmol/ml, p = 0.093). There were no differences in plasma tAM concentrations between the two groups (p = 0.055), but pericardial fluid tAM concentrations were higher in group R than in group N (p = 0.0002).
Plasma and pericardial fluid mAM concentrations
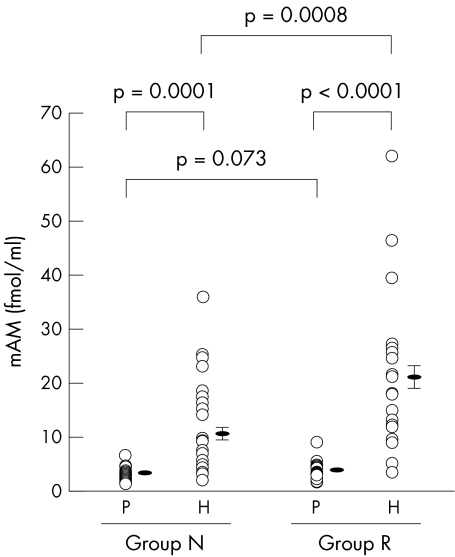
Pericardial fluid mAM concentrations were much higher than the plasma concentrations in both group N and group R (respectively, 10.6 (1.7) v 3.3 (0.2) fmol/ml, p = 0.0001; and 21.2 (2.8) v 3.9 (0.3) fmol/ml, p < 0.0001). While there were no significant differences between the two groups in plasma mAM (p = 0.073), pericardial fluid mAM was higher in group R than in group N (p = 0.0008) (fig 1).
Figure 1.
Plasma and pericardial fluid concentrations of mature adrenomedullin (mAM) in groups N and R. Group N, patients with a left ventricular end diastolic volume index of ≤ 90 ml/m2; group R, patients with a left ventricular end diastolic volume index of > 90 ml/m2; H, pericardial fluid; P, plasma.
Ratio of mAM to tAM in plasma and pericardial fluid
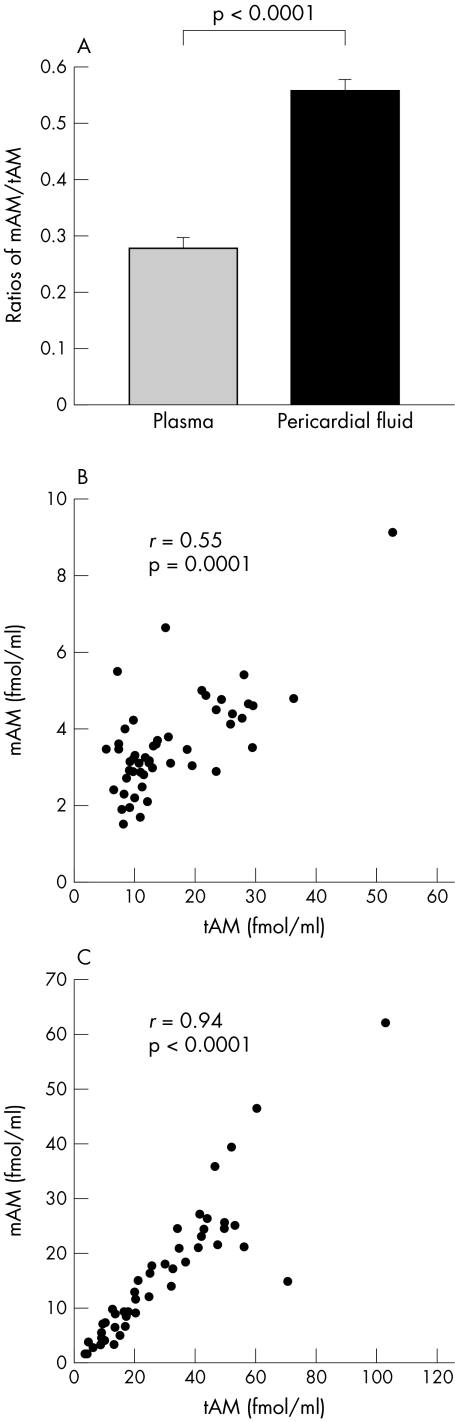
The ratio of mAM to tAM concentrations in pericardial fluid was significantly higher than in plasma (0.56 (0.02) v 0.28 (0.02), p < 0.0001) (fig 2A). Analysis using Spearman's correlation coefficient showed a moderate correlation between these concentrations in plasma and a close correlation in pericardial fluid (fig 2B and 2C). The proportional distribution of the plots in fig 2C shows that the ratios in pericardial fluid are almost constant in this patient group.
Figure 2.
The ratio of the mature form of adrenomedullin (mAM) to total immunorective adrenomedullin (tAM) in plasma and pericardial fluid (A), and the correlations between tAM and mAM coconcentrations in plasma (B) and pericardial fluid (C). r, Spearman's correlation coefficient.
Relations of plasma and pericardial fluid tAM and mAM concentrations to haemodynamic variables
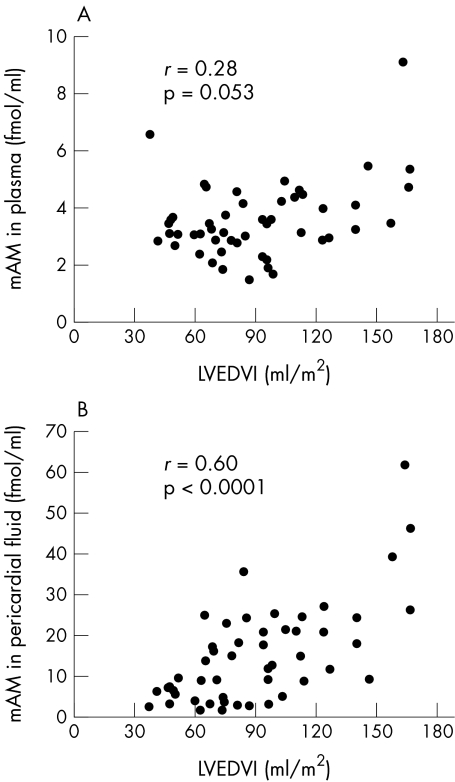
Table 2 shows correlations between plasma and pericardial fluid tAM and mAM concentrations and the left ventricular haemodynamic variables. There were no correlations with age, heart rate, or mean aortic pressure. Pericardial fluid concentrations of tAM and mAM were significantly correlated with LVEDVI (tAM, r = 0.60; mAM, r = 0.60), LVESVI (tAM, r = 0.66; mAM, r = 0.63), and LVMI (tAM, r = 0.47; mAM, r = 0.47), while plasma tAM and mAM concentrations were poorly correlated with those variables (table 2; fig 3A and 3B). Significant inverse correlations with LVEF were shown in pericardial fluid concentrations of tAM and mAM (r = −0.59, −0.54, respectively). Plasma concentrations of tAM also showed a mild inverse correlation with LVEF. No variables were correlated with LVEDP. Thus the concentrations of tAM and mAM in pericardial fluid were more closely correlated with left ventricular haemodynamic variables than the concentrations in plasma.
Table 2.
Correlations of plasma and pericardial fluid concentrations of total immunoreactive adrenomedullin (tAM) and mature form adrenomedullin (mAM) with haemodynamic variables
| tAM in plasma | tAM in pericardial fluid | mAM in plasma | mAM in pericardial fluid | |||||
| Variable | r | p Value | r | p Value | r | p Value | r | p Value |
| Age (years) | -0.094 | 0.51 | 0.004 | 0.98 | 0.15 | 0.31 | -0.065 | 0.65 |
| Heart rate (beats/min) | 0.20 | 0.17 | 0.22 | 0.12 | 0.088 | 0.54 | 0.12 | 0.42 |
| MAP (mm Hg) | -0.13 | 0.35 | -0.014 | 0.92 | 0.10 | 0.47 | -0.034 | 0.81 |
| LVEDVI (ml/m2) | 0.35 | 0.016 | 0.60 | <0.0001 | 0.28 | 0.053 | 0.60 | <0.0001 |
| LVESVI (ml/m2) | 0.41 | 0.0043 | 0.66 | <0.0001 | 0.25 | 0.078 | 0.63 | <0.0001 |
| LVEF (%) | 0.41 | 0.0044 | -0.59 | <0.0001 | -0.19 | 0.19 | -0.54 | 0.0001 |
| LVMI (g/m2) | 0.38 | 0.0082 | 0.47 | 0.0010 | 0.36 | 0.012 | 0.47 | 0.0011 |
| LVEDP (mm Hg) | 0.18 | 0.22 | 0.27 | 0.064 | 0.081 | 0.57 | 0.16 | 0.22 |
LVEDP, left ventricular end diastolic pressure; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end systolic volume index; LVMI, left ventricular mass index; MAP, mean aortic pressure.
Figure 3.
Correlations between left ventricular end diastolic volume index (LVEDVI) and concentrations of the mature form of adrenomedullin (mAM) in plasma (A) and pericardial fluid (B). r, Spearman's correlation coefficient.
DISCUSSION
It was shown very recently that immunoreactive adrenomedullin in human plasma consists of two molecular forms: mAM and iAM.5 Although various reports on the quantification of plasma adrenomedullin have been published,18–22 few have focused on mAM in relation to heart failure.6, 7 However, because the cyclic structure formed by a disulphide bond and the amidated C terminal residue of the adrenomedullin molecule are critical for its receptor binding and biological activities,3 iAM is considered to have much lower biological activity than mAM. In preliminary data, the vasodilator activity of iAM was only 5% of the activity of mAM.5 Thus, as tAM may not necessarily reflect all the activities of adrenomedullin, quantification of mAM should be performed for a full understanding of the pathophysiological role of adrenomedullin.
We and other investigators have measured pericardial fluid concentrations of various substances in cardiac patients, and some were greatly increased in comparison with the plasma concentrations.23–26 It is generally considered that pericardial fluid is not merely an ultrafiltrate of plasma, but also a transudate from the cardiac interstitium.27 In addition, a recent study suggested that the pericardium may itself release active cardiovascular mediators that function in a paracrine fashion.28 Therefore it seems possible that pericardial fluid contains higher concentrations of biologically active substances that exert local functions in the heart than does plasma. Furthermore, adrenomedullin has a molecular weight of about 6 kDa,1 which is well below the molecular weight limit for large molecules to diffuse from the cardiac interstitium into the pericardial space.27 We considered such issues when we determined the concentrations of mAM in pericardial fluid in comparison with plasma in this study.
There have been several reports that plasma concentrations of tAM are raised in patients with congestive heart failure.18–20 It has also recently been shown that plasma concentrations of mAM and iAM increase progressively with deterioration in heart failure.6, 7 In our data, however, differences in plasma concentrations of tAM and mAM between group N and group R did not reach significance. This discrepancy may reflect our selection of patients for study, where none was excluded irrespective of the presence of comorbidities that might affect plasma adrenomedullin concentrations—for example, hypertension, renal failure, inflammatory reactions, and so on.8, 21, 22 Nevertheless, pericardial fluid concentrations of tAM and mAM in group R were significantly increased over those in group N. In addition, we found that tAM and mAM in pericardial fluid correlated with indicators of left ventricular function, while plasma tAM and mAM did not. Overall, our results clearly show that pericardial fluid adrenomedullin reflects cardiac function more accurately than plasma adrenomedullin.
Our study also showed that the concentration of pericardial fluid mAM was significantly higher than plasma mAM in both groups. The ratio of the mAM concentration to the tAM concentration in pericardial fluid was twice as high as in plasma. The significantly higher concentration of mAM in pericardial fluid—not only in absolute terms but also in relation to the ratio with tAM levels—leads to the following considerations. Firstly, the heart may actively secrete mAM, which is compatible with a recent study that plasma mAM concentrations were significantly increased in the coronary sinus.29 Secondly, pericardial fluid, epicardium, pericardium, or the heart itself may have a greater capacity for enzymatic amidation than plasma, the amidation process being necessary for conversion of iAM to mAM.4
Another concept is related—that mAM functions in the heart by an autocrine or paracrine mechanism. If it works in this way, it is likely that mAM produced in the heart binds and acts in situ, and that very little is released into the blood circulation.5 There is increasing evidence from experimental models that adrenomedullin has a wide range of autocrine or paracrine functions in various organs, including inhibition of proliferation, differentiation, migration, or apoptosis of the cells.8 Concerning the heart, adrenomedullin has been shown in vitro to have direct positive inotropic effects on cardiomyocytes9 and inhibitory effects10, 11 on protein synthesis in cardiac myocytes and fibroblasts. In addition, mechanical stretching has recently been reported to stimulate mRNA expression and peptide secretion of adrenomedullin in cultured rat cardiomyocytes, which strongly supports the view that adrenomedullin participates in mechanisms opposing the cardiac hypertrophy induced by pressure and volume overload of the heart.12
While the cardioprotective functions of adrenomedullin as a local mediator have already been implicated in in vivo studies,13, 14 few have addressed human hearts. Although one report showed an association between plasma adrenomedullin concentrations and ventricular hypertrophy in patients with essential hypertension, this focused on the functions of adrenomedullin in relation to hypertension, without mentioning its autocrine or paracrine activities in the heart.30 The finding that pericardial fluid mAM was better correlated with left ventricular haemodynamic variables than plasma mAM, and that the concentrations of mAM were higher in the pericardial fluid than in plasma, both in absolute terms and as a ratio, may well reflect the autocrine or paracrine functions of adrenomedullin in the heart in patients with cardiac remodelling. The stability of the mAM/tAM ratios in pericardial fluid in our study supports this speculation as well, as it suggests the participation of a particular dominant factor causing adrenomedullin secretion into the pericardial fluid, irrespective of other secretion triggers.
Apart from the factors directly related to left ventricular remodelling, there were no significant differences in the clinical background between our two patient groups except for the numbers of patients with a history of myocardial infarction (table 1). Although this could have affected our results if old myocardial infarction gave rise not only to cardiac remodelling but also to current myocardial ischaemia, there were no differences in adrenomedullin concentrations between the patients with and without apparent ischaemia (unstable angina) in group R (data not shown). While it has been reported in various studies that tissue hypoxygenation induces the production of adrenomedullin,31, 32 mechanical stretching seemed to be a more potent stimulator of adrenomedullin secretion than hypoxia.
Limitations
We did not investigate the origins of tAM or mAM directly. It is possible that different clearance mechanisms of adrenomedullin in plasma and pericardial fluid were implicated in the higher pericardial fluid concentrations. Although the lung has been reported to be a major clearance site of circulating tAM and mAM,29, 33 the precise metabolic pathways of adrenomedullin remain to be elucidated.
Conclusions
Patients with cardiac remodelling had significantly higher concentrations of mAM in pericardial fluid than in plasma, both in absolute terms and as a ratio to tAM concentrations. Pericardial fluid mAM concentration was correlated with left ventricular haemodynamic variables, while plasma concentration was not. The biochemical characteristics of mAM and pericardial fluid suggest that adrenomedullin has autocrine or paracrine functions opposing cardiac remodelling in patients with left ventricular dysfunction.
Abbreviations
iAM, intermediate form of adrenomedullin
LVEDP, left ventricular end diastolic pressure
LVEDVI, left ventricular end diastolic volume index
LVEF, left ventricular ejection fraction
LVESVI, left ventricular end systolic volume index
LVMI, left ventricular mass index
mAM, mature adrenomedullin
tAM, total immunoreactive adrenomedullin
REFERENCES
- 1.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human phaeochromocytoma. Biochem Biophys Res Commun 1993;192:553–60. [DOI] [PubMed] [Google Scholar]
- 2.Jougasaki M, Wei CM, Aarhus LL, et al. Renal localization and actions of adrenomedullin: a natriuretic peptide. Am J Physiol 1995;268:F657–63. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi S, Hirata Y, Iwasaki H, et al. Structure–activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology 1994;135:2454–8. [DOI] [PubMed] [Google Scholar]
- 4.Cuttitta F. Peptide amidation: signature of bioactivity. Anat Rec 1993;236:87–93. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura K, Kato J, Kawamoto M, et al. The intermediate form of glycine-extended adrenomedullin is the major circulating molecular form in human plasma. Biochem Biophys Res Commun 1998;244:551–5. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama N, Kitamura K, Imamura T, et al. Molecular forms of circulating adrenomedullin in patients with congestive heart failure. J Endocrinol 1999;160:297–303. [DOI] [PubMed] [Google Scholar]
- 7.Ohta H, Tsuji T, Asai S, et al. One-step direct assay for mature-type adrenomedullin with monoclonal antibodies. Clin Chem 1999;45:244–51. [PubMed] [Google Scholar]
- 8.Jougasaki M, Burnett JC. Adrenomedullin: potential in physiology and pathophysiology. Life Sci 2000;66:855–72. [DOI] [PubMed] [Google Scholar]
- 9.Szokodi I, Kinnunen P, Tavi P, et al. Evidence for cAMP-independent mechanisms mediating the effects of adrenomedullin, a new inotropic peptide. Circulation 1998;97:1062–70. [DOI] [PubMed] [Google Scholar]
- 10.Tsuruda T, Kato J, Kitamura K, et al. Adrenomedullin: a possible autocrine or paracrine inhibitor of hypertrophy of cardiomyocytes. Hypertension 1998;31:505–10. [DOI] [PubMed] [Google Scholar]
- 11.Tsuruda T, Kato J, Kitamura K, et al. An autocrine or a paracrine role of adrenomedullin in modulating cardiac fibroblast growth. Cardiovasc Res 1999;43:958–67. [DOI] [PubMed] [Google Scholar]
- 12.Tsuruda T, Kato J, Kitamura K, et al. Enhanced adrenomedullin production by mechanical stretching in cultured rat cardiomyocytes. Hypertension 2000;35:1210–14. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihara F, Nishikimi T, Horio T, et al. Ventricular adrenomedullin concentration is a sensitive biochemical marker for volume and pressure overload in rats. Am J Physiol 2000;278:H633–42. [DOI] [PubMed] [Google Scholar]
- 14.Nagaya N, Nishikimi T, Yoshihara F, et al. Cardiac adrenomedullin gene expression and peptide accumulation after acute myocardial infarction in rats. Am J Physiol 2000;278:R1019–26. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation 1977;55:613–18. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy JW, Trenholme SE, Kasser IS. Left ventricular volume and mass from single-plane cineangiocardiogram. a comparison of anteroposterior and right anterior oblique methods. Am Heart J 1970;80:343–52. [DOI] [PubMed] [Google Scholar]
- 17.Ohta H, Tsuji T, Asai S, et al. A simple immunoradiometric assay for measuring the entire molecules of adrenomedullin in human plasma. Clin Chim Acta 1999;287:131–43. [DOI] [PubMed] [Google Scholar]
- 18.Jougasaki M, Wei CM, McKinley LJ, et al. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation 1995;92:286–9. [DOI] [PubMed] [Google Scholar]
- 19.Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol 1995;26:1424–31. [DOI] [PubMed] [Google Scholar]
- 20.Jougasaki M, Rodeheffer RJ, Redfield MM, et al. Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest 1996;97:2370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest 1994; 94:2158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishio K, Akai Y, Murao Y, et al. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit Care Med 1997;25:953–7. [DOI] [PubMed] [Google Scholar]
- 23.Fujita M, Ikemoto M, Kishishita M, et al. Elevated basic fibroblast growth factor in pericardial fluid of patients with unstable angina. Circulation 1996;94:610–13. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Hasegawa K, Fujita M, et al. Marked elevation of brain natriuretic peptide levels in pericardial fluid is closely associated with left ventricular dysfunction. J Am Coll Cardiol 1998;31:399–403. [DOI] [PubMed] [Google Scholar]
- 25.Horkay F, Laine M, Szokodi I, et al. Human pericardial fluid contains the highest amount of endothelin-1 of all mammalian biologic fluids thus far tested. J Cardiovasc Pharmacol 1995;26(suppl 3):S502–4. [PubMed] [Google Scholar]
- 26.Fazekas L, Horkay F, Kekesi V, et al. Enhanced accumulation of pericardial fluid adenosine and inosine in patients with coronary artery disease. Life Sci 1999;65:1005–12. [DOI] [PubMed] [Google Scholar]
- 27.Page E, Upshaw-Earley J, Goings G. Permeability of rat atrial endocardium, epicardium, and myocardium to large molecules: stretch-dependent effects. Circ Res 1992;71:159–73. [DOI] [PubMed] [Google Scholar]
- 28.Mebazaa A, Wetzel RC, Dodd JM, et al. Potential paracrine role of the pericardium in the regulation of cardiac function. Cardiovasc Res 1998;40:332–42. [DOI] [PubMed] [Google Scholar]
- 29.Hirayama N, Kitamura K, Imamura T, et al. Secretion and clearance of the mature form of adrenomedullin in humans. Life Sci 1999;64:2505–9. [DOI] [PubMed] [Google Scholar]
- 30.Sumimoto T, Nishikimi T, Mukai M, et al. Plasma adrenomedullin concentrations and cardiac and arterial hypertrophy in hypertension. Hypertension 1997;30:741–5. [DOI] [PubMed] [Google Scholar]
- 31.Cormier-Richard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 1998;273:17787–92. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer KH, Jensen BL, Kurtz A, et al. Tissue hypoxygenation activates the adrenomedullin system in vivo. Am J Physiol 2000;278:R513–19. [DOI] [PubMed] [Google Scholar]
- 33.Nishikimi T, Kitamura K, Saito Y, et al. Clinical studies on the sites of production and clearance of circulating adrenomedullin in human subjects. Hypertension 1994;24:600–4. [DOI] [PubMed] [Google Scholar]