The management of supraventricular tachycardia (SVT) has changed considerably over the last 10 years, and some of the techniques that interventional electrophysiologists were using last year are now outdated. This rapid evolution means that many cardiologists who do not specialise in this field find it difficult to keep up to date with the optimum strategies for the investigation and treatment of arrhythmia. This review aims to give an update on the available treatment options and their outcomes, and provide a guide to appropriate referral to specialist interventional cardiac electrophysiologists.
CLASSIFICATION AND AETIOLOGY OF SUPRAVENTRICULAR ARRHYTHMIAS
Most tachycardia has a re-entry mechanism (fig 1) and the classification of most arrhythmias is based on the location of this re-entry circuit. Tachycardia can be categorised as ventricular (involving the ventricle ± the His-Purkinje system only) and supraventricular (involving the supra-hisian structures with or without ventricular tissue). They can then be subdivided into regular or irregular tachycardia. Irregular SVTs—that is, atrial fibrillation (AF)—are less amenable to catheter ablation than regular SVT, but catheter ablation may be possible in selected patients. Regular SVTs can be cured by catheter ablation with high success rates (95–99%) and low complication rates (< 1%). Regular SVTs take the form of:
atrioventricular re-entry tachycardias (AVRT), using the ventricle as part of the circuit; these tachycardias are dependent on the presence of an accessory atrioventricular (AV) pathway (fig 2)
atrioventricular nodal re-entry tachycardia (AVNRT), where the re-entry circuit is within the AV node and the ventricle plays no part in maintaining the arrhythmia (fig 3)
atrial tachycardia, where the re-entry circuit does not involve any part of the AV junction. Examples are atrial flutter (fig 4) or ectopic atrial tachycardia (AT) (fig 5).
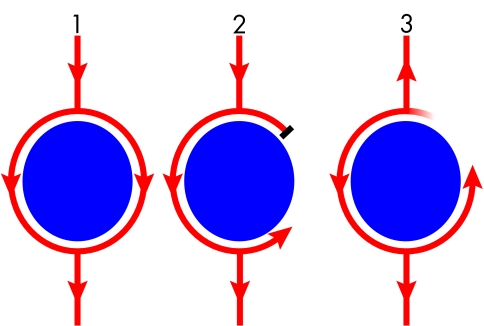
Figure 1.
Diagrammatic representation of the mechanisms of re-entry. (1) A wavefront (arrows), initiated in a normal fashion in the sinus node, passes around an obstacle (disc) to electrical activation in a uniform fashion. This obstacle may be formed by an anatomical feature (fixed conduction block) like the tricuspid annulus, or by a physiological abnormality (functional conduction block) like an area of ischaemic myocardium, which may or may not result in block depending on a variety of conditions like vagal tone or coupling interval. (2) A premature impulse results in block of conduction on one side of the obstacle while conduction continues on the other. This is functional block because it is the result of a short electrical coupling interval which means that the myocardium in this region has not recovered its excitability in time to conduct the premature beat. (3) This wavefront takes sufficient time to circulate around the obstacle that repolarisation occurs and the area previously resulting in block recovers its excitability, so that this wavefront continually encounters excitable tissue and perpetuates as a re-entry circuit.
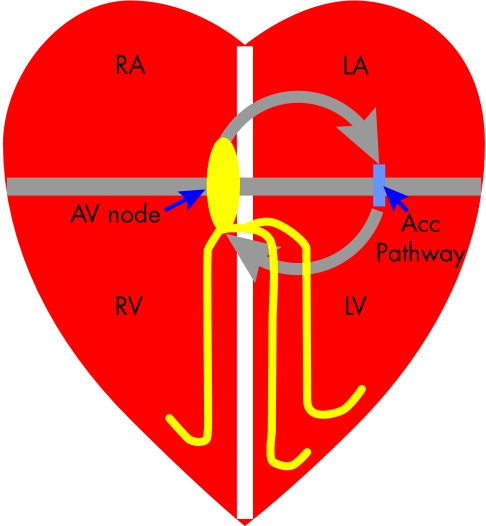
Figure 2.
Mechanism of atrioventricular re-entry tachycardias (AVRT). The right and left atria (RA/LA) and ventricles (RV/LV) are normally electrically isolated by the fibrous rings that form the mitral and tricuspid annulus (grey line), with the only connection being the atrioventricular (AV) node. If a patient has an accessory connection (acc pathway) then the requirements for a re-entry circuit (grey arrows) are fulfilled; where the mitral/tricuspid annuli form fixed conduction block and the AV node, accessory pathway and tissue of the atria and ventricles form the re-entry circuit.
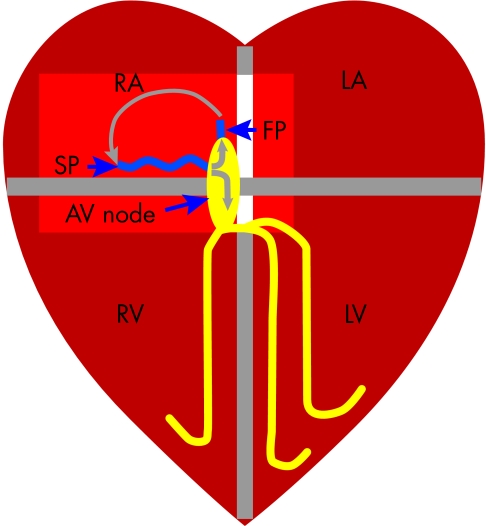
Figure 3.
Mechanism of atrioventricular nodal re-entry tachycardia (AVNRT). Patients with AVNRT have two inputs to the AV node—a slow (SP) and fast (FP) pathway. The re-entry circuit is formed by the SP, AV node, FP and the atrial tissue intervening between the FP and SP, and most commonly activates in the direction shown by the grey arrows. Note that the ventricle activates via the AV node but is not part of the re-entry circuit (unlike in AVRT). Adenosine will block conduction through the AV node, which is part of the re-entry circuit in both AVNRT and AVRT and therefore terminates the tachycardia.
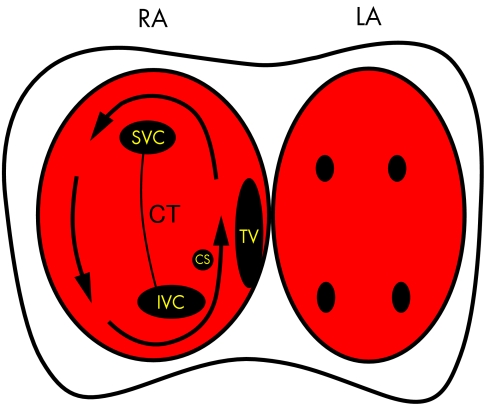
Figure 4.
Diagram showing the pathway taken by the re-entry wavefront causing atrial flutter (arrow). The right (RA) and left atria (LA) are depicted with the anatomical landmarks in the right atria marked as follows: SVC, superior vena cava; IVC, inferior vena cava; CS, coronary sinus os; TV, tricuspid valve; CT, crista terminalis. The TV and CT form lines of conduction block which “electrically” divide the atria into two halves. The only electrical connections between these two halves are the roof and appendage of the right atrium and the isthmus between the TV and IVC. Note that the narrowest part of the re-entry circuit is this isthmus which is why this is the target for ablation of atrial flutter. The left atrium activates via conduction through the septum but is not part of the re-entry circuit.
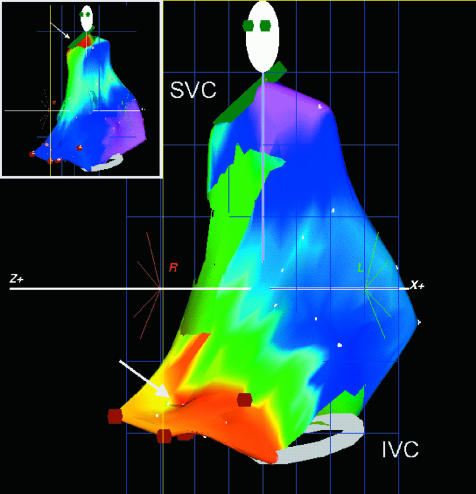
Figure 5.
An electroanatomical map of the right atrium in a patient with focal atrial tachycardia (AT). The atrium is shown in the left anterior oblique (LAO) view with the superior (SVC) and inferior vena cava (IVC) shown as rings on the map. An isochronal map has been superimposed onto the computer generated model of the right atrium. This shows early activation in red and latest activation in purple. The earliest activation can be seen at the low lateral right atrium which is in contrast to activation during sinus rhythm (inset) which is earliest at the superior lateral right atrium, the location of the sinus node.
MECHANISMS OF SVTs
Atrioventricular re-entry tachycardias
Patients with AVRT are born with an accessory pathway which usually has very different conduction characteristics from the AV node. As a result these tachycardias may present at any age, even in neonates or early childhood.
Atrioventricular nodal re-entry tachycardia
AVNRT, however, is dependent on the difference in conduction properties of the two atrial inputs into the AV node—the fast and slow pathway.1 AVNRT, the most common regular SVT, often presents in early adulthood, probably because maturation of the AV node results in differentiation of the conduction properties of the fast and slow pathways.
Atrial flutter
As with all tachycardias with a re-entry mechanism, atrial flutter is dependent on the passage of the activation wave front around the atrium taking long enough to allow repolarisation of myocardium before the wave front completes one circuit, so that the wave front is always approaching excitable myocardium. This may happen as a result of slow conduction velocity or a long wave front path.2–4 Stretching and scarring of the atria are likely to produce these conditions and so patients with atrial flutter present at an older age and often have concomitant disease predisposing to atrial pathology, such as atrial septal defect, previous surgery or ischaemic heart disease.
Atrial tachycardia
AT is now considered a misnomer and really refers to focal AT as opposed to atrial flutter and fibrillation.5 It is rare and has a focal mechanism which has not been clearly defined5; indeed, it may be that the cause is a very small re-entry circuit, but the mapping systems used to characterise the circuit do not have adequate resolution.
Atrial fibrillation
AF, the irregular SVT, is the most common and results from multiple re-entry circuits which can have varying degrees of randomness, so that the tachycardia may appear relatively organised or disorganised on the 12 lead ECG.6 The re-entry is established by lines of conduction block resulting from scarring, ischaemia or stretch of the atria, and therefore AF may often be associated with hypertension, mitral valve disease, and ischaemic heart disease.7
INCIDENCE
The incidence of regular SVT in the population has been poorly defined. AVNRT is by far the most common regular SVT and accounts for 90% of so called “junctional tachycardias”,8 the remainder being dependent on the presence of an accessory AV connection to produce AVRT. Epidemiological studies have demonstrated an incidence of regular AVNRT or AVRT of 35/100 000 person-years and a prevalence of 2.29/1000 persons.9 Atrial flutter has an incidence of 88/100 000 person-years and is unsurprisingly associated with increasing age, patients over 80 years having an incidence of 587/100 000.10 AF is the most common sustained cardiac arrhythmia,11 having a prevalence of 6% in the population over 65 years old,11 and is the most common cardiac cause of stroke.12 Because of this AF represents a considerable burden on health care systems which is why enormous efforts are currently being made to develop curative treatments.
TREATMENTS FOR SVT
Palliation
Antiarrhythmic drugs act by either slowing conduction or lengthening the refractory period of cardiac tissue. Some antiarrhythmic drugs have selective actions. An example is flecainide, which has some effect on the AV node, prolonging the effective refractory period by 10% and the conduction velocity by 20%13, 14; by contrast, it will completely block anterograde conduction in accessory pathways in approximately 40% of patients while prolonging refractory periods in 20% of the remaining cases.15, 16 For the majority of SVT pharmacological treatment results in changes to the conduction properties of all or a large proportion of cardiac conduction tissue. Therefore, when prescribing these treatments, the physician hopes that the drug will either: (a) change the conduction velocity of components of the re-entry circuit differentially, so that they no longer differ sufficiently to allow re-entry to be established; or (b) that the refractory period is lengthened so that the re-entry wave front encounters refractory tissue and is therefore extinguished. If conduction velocity is slowed in all parts of the circuit equally, or the refractory period of one part of the circuit is lengthened by a critical amount, this can be proarrhythmic, and antiarrhythmic drugs may therefore result in incessant tachycardia. A brief synopsis of drug treatment of SVT is shown in the box below.
AVRT/AVNRT.
Recurrence rate on antiarrhythmic drugs is around 20%9
- Useful drugs:
- (a) AVRT: flecainide, disopyramide or β blockers
- (b) AVNRT: verapamil, β blockers, sotalol, flecainide or disopyramide
Atrial flutter.
Recurrence rate: 55%, 6 months after DC cardioversion17 and as high as 60% long term, even with antiarrhythmic treatment18
Antiarrhythmic drugs that slow atrial conduction and lengthen refractory period (for example, flecainide, amiodarone) may have some effect on reducing atrial flutter recurrence, but drugs that reduce rate may also slow the flutter enough to allow 1:1 AV nodal conduction, resulting in a tachycardia rapid enough to cause haemodynamic compromise19
Alternatively, antiarrhythmic drug strategies for atrial flutter may aim to control the ventricular response rather than maintain sinus rhythm. Because the ventricular response rate is a function of the atrial flutter rate, achieving a balance between tachycardia (2:1 AV block or 3:1 AV block causing ventricular response rates of 150 and 100 beats per minute) and bradycardia is difficult, and many opt to encourage AF and control rate response with digoxin and β blockers
Atrial fibrillation.
Curative therapies
Catheter ablation is a minimally invasive procedure whereby 4–8 French electrodes are passed intravascularly, most commonly to the right heart, under x ray guidance. The procedure is performed under local anaesthetic, often as a day case procedure. The general principles of catheter ablation are that an electrophysiological study (EPS) is performed first using a specifically designed pulse generator to perform programmed stimulation. The primary aim of the EPS is to induce tachycardia so that the activation sequence recorded by the diagnostic catheter electrodes can be used to determine the arrhythmia mechanism. A deflectable mapping/ablation catheter is then positioned on a portion of the re-entry circuit critical for maintenance of tachycardia that is not part of the normal cardiac conduction system. Once in a suitable location, radiofrequency (RF) energy is delivered via the ablation catheter. This results in conductive and resistive heating of the endocardium and some of the myocardium rendering a small area (2–4 mm3) electrically inactive. Most patients do not feel the energy application but some patients feel warmth or even pain in the chest during RF application. More often, patients find lying on the x ray table for the length of time required for the ablation more troublesome than the actual procedure itself; although this may result in suppression of the arrhythmia, light sedation or analgesics can help. After RF delivery, a further limited EPS is then performed to confirm successful ablation of the re-entry circuit. The targets, success rates, and complication rates for each SVT are summarised in table 1.
Table 1.
Results of radiofrequency catheter ablation
| Arrhythmia | Success rate (%) | Complication rate (%) | Possible additional/ maintenance therapy |
| AVNRT | 98 | 0–2 | None |
| AVRT | 99 | 1.8 | None |
| Atrial flutter | 95 | <1 | None |
| AV node ablation for established AF | >99 | <1 | Permanent pacemaker |
| AF secondary to focal tachycardia | 62 | 5 | Antiarrhythmic drugs |
This table shows the outcome of catheter ablation for arrhythmia. Some of these data are likely to be out of date; an example is focal atrial fibrillation in which the techniques are progressing rapidly enough that published data are not applicable to currently used approaches and are likely to underestimate the success rates.
AF, atrial fibrillation; AV, atrioventricular; AVNRT, atrioventricular nodal re-entry tachycardia, AVRT, atrioventricular re-entry tachycardia
Complication rates vary depending on the arrhythmia being ablated. Estimated rates for individual complications are listed in table 2, based on prospective data collected in the USA in 1998,23 apart from ablation of AF secondary to focal tachycardia for which data have not yet been published (this is estimated from the data from our centre and that associated with left sided accessory pathway ablation). For newer procedures—that is, AF ablation—some of these data may already be outdated. The extra risk of developing a cancer over a lifetime from the radiation exposure associated with an hour of fluoroscopy is 480 per million patients.24 Few ablation procedures require this degree of exposure; the doses received by patients undergoing even the most complex catheter ablation have been reduced by modern fluoroscopy equipment or non-fluoroscopic cardiac mapping systems.
Table 2.
Major complications associated with ablation procedures
| Arrhythmia | Heart block (%) | Vascular damage/ haematoma (%) | Other (%) | Death (%) |
| AVNRT | 0.9 | 0.49 | 0.08 DVT | 0 |
| 0.08 PE | ||||
| 0.08 Pneumothorax | ||||
| AVRT: left sided | <0.1 | 3.1 | 1.7 Tamponade | 0 |
| 0.2 Coronary occlusion | ||||
| AVRT: right free wall | 1 (3rd degree) | 0 | 1 PE | 0 |
| AVRT: septal | 0.5 | 0.5 | 0.5 Pneumothorax | 0 |
| 0.5 Pericarditis | ||||
| Atrial flutter | 0.4 | 0.6 | 0.2 Tamponade | 0 |
| 0.2 DVT | ||||
| 0.2 Haemothorax | ||||
| 0.2 TR | ||||
| AV node ablation for established AF | N/A | 0.3 | 0.15 TR | 0.15 (pacemaker failure) |
| AF secondary to focal tachycardia | 0 | 1 | 2 Tamponade 0.5 Embolic stroke | 0 |
DVT, deep vein thrombosis; PE, pulmonary embolus, TR, tricuspid regurgitation. For key to rest of abbreviations see table 1.
GENERAL GUIDANCE FOR REFERRAL
Regular SVT
The studies comparing catheter ablation with drug treatment are limited because it has been generally accepted for some years that catheter ablation has greater efficacy and the evolution of techniques for catheter ablation has been so rapid. Studies have shown that catheter ablation has a higher success rate than drug treatment,25, 26 patients have a higher quality of life after catheter ablation,27 and it is more cost effective than antiarrhythmic drug treatment within 9–12 years.25, 28 Most patients warrant treatment for their arrhythmia if they find the symptoms debilitating or require recurrent attendance at hospital for termination of tachycardia. If a patient has evidence of pre-excitation and a history of tachycardia, I would offer them catheter ablation even without recurrent symptoms because of the low but definite risk of sudden death associated with AF in this group of patients.29 Whether patients who have pre-excitation discovered incidentally should be offered ablation is more controversial, but EPS to assess the Wenckebach cycle length of the accessory pathway (to determine its potential for rapid conduction if AF occurs) may be misleading because a variety of factors affect the refractory period which may change from one day to the next. The case for catheter ablation of atrial flutter is even more compelling because the catheter ablation success rate is now considerably higher than drug treatment. In summary, all patients with regular SVT who require treatment should be referred to a cardiac electrophysiologist for consideration of catheter ablation.
Atrial fibrillation
The techniques used and the success rates of catheter ablation of AF are changing so rapidly that within one year this review will almost certainly be out of date in this regard. There are two approaches to catheter treatment of AF. The first is to control ventricular response rate by modifying or abolishing AV nodal conduction. AV node modification is a technique whereby the atrial inputs into the AV node with the shortest refractory periods are selectively ablated. This reduces the rate at which the AV node can conduct to the ventricle. It is a technique that has been less in vogue in recent years mainly because it is difficult to predict the outcome reliably, and patients often require pacemaker implantation because of bradycardia or repeat ablation procedures because of tachycardia. Complete AV node ablation and permanent pacemaker implantation is more frequently employed when drug treatment fails to control the ventricular response rate.
The second approach to treatment of AF, used more often for paroxysmal than established AF, involves catheter ablation of the focal activity that initiates AF. This focal activation often arises from one of the pulmonary veins and electrical isolation of these veins may cure AF in up to 60% of patients long term.30
The complication rate of this procedure is slightly higher than for most catheter ablations because a transeptal puncture is required and the catheters are passed to the left heart. This is only a marginal risk because most interventional electrophysiologists are experienced in transeptal puncture, and transoesophageal echocardiography is performed immediately before the procedure to exclude thrombus in the left atrium that may be embolised by catheter manipulation. Patients may benefit from the opinion of an interventional electrophysiologist if they are in established AF and their ventricular rate cannot be controlled with antiarrhythmic drugs, or they have lone paroxysmal AF that cannot be abolished with class Ic antiarrhythmic treatment. Amiodarone may be a suitable alternative in this group of patients, but the side effect profile of amiodarone means that other options should at least be explored before committing the patient to lifelong treatment.
COMMON MISCONCEPTIONS
(1) Flecainide causes sudden death: Although flecainide was associated with an increased risk of sudden death in CAST (cardiac arrhythmia suppression trial)31 in patients with impaired left ventricular function and ventricular premature beats, there is no evidence that it increases risk of sudden death when used in patients with SVT (and normal ventricles).32
(2) Digoxin is good at rate control: Digoxin appears to be less effective than other agents at controlling ventricular rate in AF, particularly during exercise or critical illnesses.33, 34 Moreover, in patients with paroxysmal AF, digoxin does not appear to have a significant effect on ventricular rate during paroxysms and may be associated with the occurrence of longer paroxysms.35
(3) Ablation of SVT is time consuming: Most catheter ablations take approximately 30 minutes to one hour. Factors that may prolong the procedure are difficulty in inducing the tachycardia, multiple accessory pathways, or large, abnormal atria requiring unusual mapping/ablation catheters.
(4) Ablation of SVT is traumatic: Most catheter ablation can be performed as a day case procedure and patients require no special follow up after the procedure. Although sedation may suppress the arrhythmia it will often alleviate the anxiety experienced by the patient before and during the procedure.
(5) Ablation should be reserved for patients failing antiarrhythmic drugs: Ablation for most SVT has such a low complication rate and high success rate that it is more cost effective and may be safer than antiarrhythmic drugs.
Supraventricular tachycardias: key points.
-
The following groups of patients may benefit from referral to a cardiac electrophysiologist:
(1) All patients with regular SVT on antiarrhythmic drugs
(2) All patients with recurrent regular SVT
(3) Patients with established AF in whom control of the ventricular response is suboptimal
(4) Patients with lone paroxysmal AF in whom maintenance of sinus rhythm cannot be achieved with class Ic antiarrhythmics
It is likely that an effective curative catheter ablation procedure for established AF will be established within the next 10 years
CONCLUSION
Almost all regular SVTs can be treated simply and quickly with catheter ablation. Exceptions are those that occur as a result of structural abnormalities of the atria. The treatment of AF is evolving rapidly and at the present time the only patients that may be reliably and effectively treated are those with short frequent paroxysms of AF. Electrophysiologists may still have a role in ablating established AF if control of the ventricular response is proving difficult.
Abbreviations
AF: atrial fibrillation
AT: atrial tachycardia
AV: atrioventricular
AVNRT: atrioventricular nodal re-entry tachycardia
AVRT: atrioventricular re-entry tachycardia
EPS: electrophysiological study
RF: radiofrequency
SVT: supraventricular tachycardia
REFERENCES
- 1.Jackman WM, Beckman KJ, McClelland JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med 1992;327:313–8. ▸ This is the first description of the technique of slow pathway ablation for treating AVNRT, one of the more common regular SVTs. It proved to be easier to perform and have fewer complications than the previous approach of fast pathway ablation, and as such revolutionised the treatment of patients with AVNRT. [DOI] [PubMed] [Google Scholar]
- 2.Tai CT, Chen SA, Chiang CE, et al. Characterization of low right atrial isthmus as the slow conduction zone and pharmacological target in typical atrial flutter. Circulation 1997;96:2601–11. [DOI] [PubMed] [Google Scholar]
- 3.Shah DC, Jaïs P, Haïssaguerre M, et al. Three-dimensional mapping of the common atrial flutter circuit in the right atrium. Circulation 1997;96:3904–12. [DOI] [PubMed] [Google Scholar]
- 4.Kinder C, Kall J, Kopp D, et al. Conduction properties of the inferior vena cava-tricuspid annular isthmus in patients with typical atrial flutter. J Cardiovasc Electrophysiol 1997;8:727–37. [DOI] [PubMed] [Google Scholar]
- 5.Saoudi N, Cosio F, Waldo A, et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the working group of arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol 2001;2:852–66 . [DOI] [PubMed] [Google Scholar]
- 6.Schilling RJ, Kadish AH, Peters NS, et al. Endocardial mapping of atrial fibrillation in the human right atrium using a non-contact catheter. Eur Heart J 2000;21:550–64. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Abbott RD, Savage DD. Coronary heart disease and atrial fibrillation: the Frammingham study. Am Heart J 1978;106:389–96. [DOI] [PubMed] [Google Scholar]
- 8.Josephson M, Buxton A, Marchlinski F. The tachyarrhythmias. In: Isselbacher K, Braunwald E, Wilson JD, et al, eds. Harrison's principles of internal medicine, 13th ed. New York: McGraw-Hill, 1994:1024–9.
- 9.Orejarena LA, Vidaillet H Jr, DeStefano F, et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol 1998;31:150–7. ▸ This is one of the few studies describing the epidemiology of SVT. [DOI] [PubMed] [Google Scholar]
- 10.Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol 2000;36:2242–6. [DOI] [PubMed] [Google Scholar]
- 11.Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution and gender of patients with atrial fibrillation. Arch Intern Med 1995;155:469–73. [PubMed] [Google Scholar]
- 12.Wellens HJ. Atrial fibrillation - the last big hurdle in treating supraventricular tachycardia. N Engl J Med 1994;331:944–5. [DOI] [PubMed] [Google Scholar]
- 13.Estes NAM, Garan H, Ruskin JN. Electrophysiologic properties of flecainide acetate. Am J Cardiol 1984;53(suppl B):26B–29B. [DOI] [PubMed] [Google Scholar]
- 14.Hellenstrand KJ, Bexton RS, Nathan AW, et al. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in men. Br Heart J 1982;48:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellenstrand KJ, Nathan AW, Bexton RS, et al. Electrophysiological effects of flecainide acetate on sinus node function anomalous atrioventricular connections and pacemaker thresholds. Am J Cardiol 1984;53:30–8. [DOI] [PubMed] [Google Scholar]
- 16.Neuss H, Buss J, Schlepper M, et al. Effects of flecainide on electrophysiological properties of accessory pathways in the Wolff-Parkinson-White syndrome. Eur Heart J 1983;4:347–53. [DOI] [PubMed] [Google Scholar]
- 17.Pozen RG, Pastoriza J, Rozanski JJ, et al. Determinants of recurrent atrial flutter after cardioversion. Br Heart J 1983;50:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pozen RG, Pastoriza J, Rozanski JJ, et al. Determinants of recurrent atrial flutter after cardioversion. Am J Cardiol 1993;71:710–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan AW, Hellestrand KJ, Bexton RS, et al. Proarrhythmic effects of the new antiarrhythmic agent flecainide acetate. Am Heart J 1984;107:222–8. [DOI] [PubMed] [Google Scholar]
- 20.Chun SH, Sager PT, Stevenson WG, et al. Long-term efficacy of amiodarone for the maintenance of normal sinus rhythm in patients with refractory atrial fibrillation or flutter. Am J Cardiol 1995;76:47–50. [DOI] [PubMed] [Google Scholar]
- 21.Horowitz LN, Spielman SR, Greenspan AM, et al. Use of amiodarone in the treatment of persistent and paroxysmal atrial fibrillation resistant to quinidine therapy. J Am Coll Cardiol 1985;6:1402–7. [DOI] [PubMed] [Google Scholar]
- 22.Gold RL, Haffajee CI, Charos G, et al. Amiodarone for refractory atrial fibrillation. Am J Cardiol 1986;57:124–7. [DOI] [PubMed] [Google Scholar]
- 23.Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. PACE 2000;23:1020–8. ▸ This is the first prospective study to describe the outcome of catheter ablation using modern techniques in large numbers of patients and centres. Using the data provided by this study may be less misleading to patients because the complications of catheter ablation are so rare it is not uncommon for individual centres to have fewer than one major complication a year.10879389 [Google Scholar]
- 24.Perisinakis K, Damilakis J, Theocharopoulos N, et al. Accurate assessment of patient effective radiation dose and associated detriment risk from radiofrequency catheter ablation procedures. Circulation 2001;104:58–62. [DOI] [PubMed] [Google Scholar]
- 25.Weerasooriya HR, Murdock CJ, Harris AH, et al. The cost-effectiveness of treatment of supraventricular arrhythmias related to an accessory atrioventricular pathway: comparison of catheter ablation, surgical division and medical treatment. Aust NZ J Med 1994;24:161–7. [DOI] [PubMed] [Google Scholar]
- 26.Natale A, Newby KH, Pisano E, et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol 2000;35:1898–904. [DOI] [PubMed] [Google Scholar]
- 27.Bathina MN, Mickelsen S, Brooks C, et al. Radiofrequency catheter ablation versus medical therapy for initial treatment of supraventricular tachycardia and its impact on quality of life and healthcare costs. Am J Cardiol 1998;82:589–93. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda T, Sugi K, Enjoji Y, et al. Cost effectiveness of radiofrequency catheter ablation versus medical treatment for paroxysmal supraventricular tachycardia in Japan. J Cardiol 1994;24:461–8. [PubMed] [Google Scholar]
- 29.Fitzsimmons PJ, McWhirter PD, Peterson DW, et al. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J 2001;142:530–6. ▸ This describes the true long term outcome of unselected patients with Wolff-Parkinson-White (WPW) syndrome. These data are particularly relevant to asymptomatic patients with WPW detected at health screening, which raises the dilemma as to whether catheter ablation of this potentially life threatening condition should be performed. In fact this study shows that sudden cardiac death in patients with WPW is rare. [DOI] [PubMed] [Google Scholar]
- 30.Haissaguerre M, Jais P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 2000;101:1409–17. ▸ This is one of the first descriptions of the currently employed technique for eliminating the focal triggers for paroxysmal atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 31.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989;321:406–12 . [DOI] [PubMed] [Google Scholar]
- 32.Pritchett ELC, Wilkinson WE. Mortality in patients treated with flecainide and encainide for supraventricular arrhythmias. Am J Cardiol 1991;67:976–80. [DOI] [PubMed] [Google Scholar]
- 33.Goldman S, Probst P, Selzer A, et al. Inefficacy of “therapeutic” serum levels of digoxin in controlling the ventricular rate in atrial fibrillation. Am J Cardiol 1975;35:651–5. [DOI] [PubMed] [Google Scholar]
- 34.Botker HE, Toft P, Klitgaard NA, et al. Influence of physical exercise on serum digoxin concentration and heart rate in patients with atrial fibrillation. Br Heart J 1991;65:337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawles JM, Metcalfe MJ, Jennings K. Time of occurrence, duration, and ventricular rate of paroxysmal atrial fibrillation: the effect of digoxin. Br Heart J 1990;63:225–7. [DOI] [PMC free article] [PubMed] [Google Scholar]