Abstract
Background: The design of devices currently used for closure of persistent ductus arteriosus (PDA) with high pulmonary artery pressure is not ideal and there is a risk of embolisation into the aorta.
Objective: To investigate the use of the Amplatzer muscular ventricular septal defect occluder (AMVSDO) for treatment of PDA with high pulmonary artery pressure.
Patients and design: Seven patients, aged 5–12 years, with large PDAs and systemic or near systemic pulmonary artery pressure underwent attempted transcatheter closure using the AMVSDO. The device consists of two low profile disks made of a nitinol wire mesh with a 7 mm connecting waist. Balloon occlusion of the duct was performed before closure from the venous side, and prosthesis size was chosen according to the measured diameter of the occluding balloon. A 7 French sheath was used to deliver the device. All patients underwent a complete haemodynamic and angiographic study one year after occlusion.
Results: The mean (SD) angiographic PDA diameter was 9.8 (1.7) mm (range 7–13 mm) and the mean AMVSDO diameter was 11.4 (1.8) mm (range 9–16 mm). Qp/Qs ranged from 1.9–2.2 (mean 2.0 (0.1)). Successful device delivery and complete closure occurred in all patients (100% occlusion rate, 95% confidence interval 59.04% to 100.00%). Mean systolic pulmonary artery pressures were as follows: before balloon occlusion, 106 (13) mm Hg; during occlusion, 61 (6) mm Hg; immediately after the procedure, 57 (5) mm Hg; and at the one year follow up catheterisation, 37 (10) mm Hg. Fluoroscopy time was 10.4 (4.3) min (range 7–18 min). No complications occurred.
Conclusions: AMVSDO is an important adjunct for closure of large PDAs associated with high pulmonary artery pressure. Further studies are required to document its efficacy, safety, and long term results in a larger number of patients.
Keywords: AMVSDO, Amplatzer muscular ventricular septal defect occluder, persistent ductus arteriosus, pulmonary hypertension, Amplatzer VSD occluder
Transcatheter closure of persistent ductus arteriosus (PDA) using various occluders and coils is a well established method.1–7 However, not all patients with PDA are amenable to this type of treatment, as these devices—including the new Amplatzer duct occluder8–10—are not suitable for large high pulmonary artery pressure ducts (HPAP-PDA). In the presence of high pulmonary artery pressure such devices carry the risk of embolising into the aorta. The Amplatzer muscular ventricular septal defect occluder (AMVSDO) is a new device which has recently been used successfully for transcatheter closure of muscular ventricular septal defects.11, 12 This occluder, which is available in a variety of sizes, may be more suitable for use with HPAP-PDA as its double disk tends to anchor the device, preventing embolisation into the aorta. In this study we report the successful use of the AMVSDO for treating HPAP-PDA.
METHODS
Device design
The AMVSDO (AGA Medical Corporation, Golden Valley, Minnesota, USA) has been described in detail in previous reports.11, 12 In brief, the AMVSDO is a self centering and repositionable device constructed of 0.004 inch (0.1 mm) Nitinol wires, tightly woven into two flat round discs with a 7 mm connecting waist. The left disc is 4 mm larger than the waist and the right disc is 3 mm larger than the waist. Prostheses are currently available in sizes (waist diameters) ranging from 4–24 mm. The device is delivered through a long 6–8 French sheath.
Patient population
From May 1998 to August 2000, seven patients with clinical and echocardiographic findings of a large PDA and pulmonary hypertension underwent transcatheter closure with the AMVSDO. Their median age was 9 years (range 5–12 years) and their median body weight was 26 kg (19–57 kg). Three patients had symptoms of heart failure or failure to thrive. On cross sectional and Doppler echocardiography there was evidence of bidirectional shunting through the PDA with left atrial and left ventricular enlargement.
Informed parental consent for the procedures was obtained in each patient.
Procedure
The technique of transcatheter closure of PDA using the AMVSDO was similar to that described by Masura and colleagues with the use of the Amplatzer duct occluder.8 After percutaneous puncture of the femoral artery and vein, a complete haemodynamic evaluation was performed with pressure and saturation measurements taken in all cardiac chambers. A biplane descending aortogram in anteroposterior and lateral projections was performed with a 5 or 6 French pigtail catheter to define the size and anatomy of the PDA (fig 1, panel 1) Pulmonary artery and aortic pressure measurements were repeated during balloon duct occlusion, which was performed from the venous side using an 8 French Berman angiographic catheter or a Meditech sizing balloon (Meditech, Watertown, Massachusetts, USA). In addition to echocardiographic evidence (left atrial and left ventricular enlargement) and haemodynamic findings (ratio of pulmonary to systemic blood flow (Qp/Qs) ≥ 1.9:1) indicating a significant left to right shunt, a fall in the systolic pulmonary artery pressure of more than 30% during balloon occlusion was our criterion for proceeding to transcatheter closure. Subsequently, a 5 French Cobra type catheter was advanced percutaneously from the venous side through the PDA into the descending aorta. Using an exchange 260 cm, 0.035 inch guide wire, the cobra catheter was exchanged for a 7 French delivery sheath which was advanced directly through the femoral vein and positioned in the proximal descending aorta.
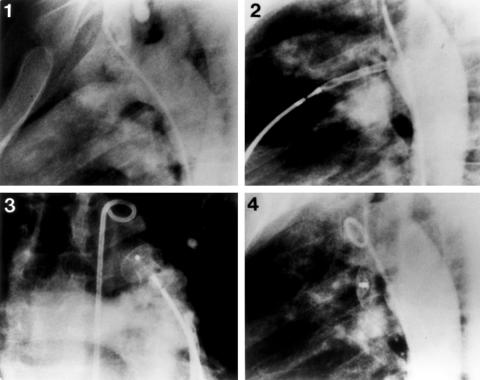
Figure 1.
Steps in the transcatheter closure technique with the Amplatzer muscular ventricular septal defect occluder (AMVSDO) in an 11 year old patient with a large high pulmonary artery pressure persistent ductus arteriosus (PDA) (patient 3). (1) Descending aortogram in the lateral projection showing a large PDA measuring 10 mm in diameter. (2) Deployment of the retention disc of a 12 mm AMVSDO opposite the orifice of the duct. (3) Pulmonary arteriogram in the cranial projection showing good position of the device with no obstruction of the pulmonary artery. (4) Descending aortogram 10 minutes after release of the device, revealing complete closure with good device position.
An appropriately sized occluder (waist diameter equal to the balloon stretched PDA diameter) was screwed to the delivery cable, pulled into the loader, and introduced into the guiding sheath. Under fluoroscopic guidance, the occluder was advanced into the descending aorta, where the left disk was deployed and pulled gently against the orifice of the duct (fig 1, panel 2). Correct position was confirmed by injection of contrast medium through the aortic catheter into the descending aorta. Using gentle tension on the delivery cable, the sheath was pulled back to deploy the rest of the device. With the device still attached to the delivery cable, cross sectional colour Doppler echocardiography, pulmonary arteriography (fig 1, panel 3), and descending aortography (hand injection of contrast medium) were done to confirm proper device position and exclude left pulmonary or aortic obstruction. Once optimal position was confirmed, the AMVSDO was released by counterclockwise rotation of the delivery cable. A repeat aortogram (fig 1, panel 4) and a complete haemodynamic evaluation were performed to check for residual shunts and change of pressures. Prophylactic antibiotics were not routinely given during the procedure. All patients were sent home 24 hours after the procedure on no drug treatment. Endocarditis prophylaxis was discontinued at the 12 month follow up visit if the duct was completely closed.
Follow up
A chest x ray and complete cross sectional and colour Doppler echocardiographic studies were performed on all patients at 24 hours, one month, and serially at 3–6 month intervals. In addition, all patients underwent a complete haemodynamic and angiographic study one year after the transcatheter closure.
Statistical analysis
Results were analysed with the Stratgraphic statistical program and expressed as mean (SD), with confidence intervals where applicable. Preocclusion and postocclusion data were compared using the paired Wilcoxon test. A probability value of p < 0.05 was considered significant.
RESULTS
The clinical and haemodynamic data before and after occlusion, and the outcome in the seven patients, are shown in table 1. According to Krichenko's PDA classification,13 five patients had type A, one had type C, and one had type E. The length of the duct varied between 6–8 mm (mean 7.2 (0.5) mm). The mean duct diameter (pulmonary end) was 9.8 (1.7) mm (range 7–13 mm). The mean AMVSDO diameter was 11.4 (1.8) mm (9–16 mm). The pulmonary to systemic flow ratio (Qp/Qs) varied between 1.9–2.2 (mean 2.04 (0.14)). Patient 7 had a perimembranous ventricular septal defect 6 mm in size in addition to the PDA. All patients had systemic or near systemic systolic pulmonary artery pressure (mean 106 (13) mm Hg; mean systolic aortic pressure 112 (9) mm Hg). Device delivery was successful and associated with complete closure in all seven patients (100% closure rate, 95% confidence interval 59.04% to 100%). There was a significant fall (p < 0.05) in mean systolic pulmonary artery pressure during balloon occlusion (to 61 (6) mm Hg) and immediately after the placement of the AMVSDO (to 57 (5) mm Hg). Fluoroscopy time was 10.4 (4.5) minutes (range 7–18 minutes). No patient experienced any complication as a result of the procedure.
Table 1.
Clinical and haemodynamic data
| Before closure | After closure | Follow up catheterisation | ||||||||||
| Patient No/age (years) | Weight (kg) | Duct diameter (mm) | AMVSDO diameter (mm) | SPAP 1 (mm Hg) | SPAP 2 (mm Hg) | SAOP (mm Hg) | SPAP (mm Hg) | SAOP (mm Hg) | Results | SPAP (mm Hg) | SAOP (mm Hg) | Outcome |
| 1/12 | 57 | 13 | 16 | 130 | 70 | 130 | 60 | 120 | CC | 45 | 130 | CC |
| 2/9 | 21 | 12 | 14 | 120 | 70 | 120 | 65 | 120 | CC | 60 | 110 | CC |
| 3/11 | 39 | 10 | 12 | 100 | 55 | 110 | 60 | 120 | CC | 35 | 120 | CC |
| 4/8 | 23 | 10 | 12 | 95 | 60 | 105 | 55 | 100 | CC | 30 | 115 | CC |
| 5/7 | 26 | 11 | 14 | 100 | 60 | 110 | 55 | 105 | CC | 50 | 120 | CC |
| 6/10 | 37 | 10 | 12 | 100 | 55 | 100 | 55 | 100 | CC | 30 | 130 | CC |
| 7/5 | 19 | 7 | 9 | 100 | 60 | 115 | 50 | 110 | CC | 25 | 100 | CC |
| Mean (SD) values | ||||||||||||
| 8.9 (2.4) | 31.7 (13.6) | 9.8 (1.7) | 11.4 (1.8) | 106 (13) | 61 (6) | 112 (10) | 57 (5) | 110 (9) | 37 (9) | 117 (10) | ||
| p<0.05 | p<0.05 | p<0.05 | ||||||||||
p Values relate to comparisons with SPAP 1.
AMVSDO, Amplatzer muscular ventricular septal defect occluder diameter; CC, complete closure; SAOP, systolic aortic pressure; SPAP 1, systolic pulmonary artery pressure before balloon occlusion; SPAP 2, systolic pulmonary artery pressure during balloon occlusion.
Follow up
There was a further fall (p < 0.05) in the mean systolic pulmonary artery pressure (to 37 (9) mm Hg) at the one year follow up cardiac catheterisation. No complications were observed in the early postprocedural period or during the one year follow up. All patients had complete closure with no evidence of device recanalisation, migration, wire fracture, thromboembolism, or endocarditis (fig 2). No obstruction of the left pulmonary artery or the aorta was noted. The patient with the PDA and the perimembranous ventricular septal defect had successful surgical closure of the latter one month after transcatheter closure of the duct.
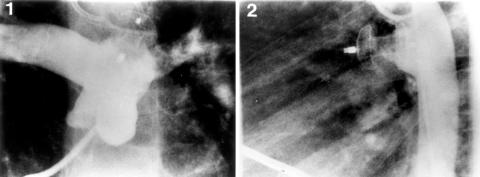
Figure 2.
Pulmonary arteriogram (1) and descending aortogram (2) one year after implantation of the AMVSDO, showing complete closure and good position of the device with no evidence of aortic or pulmonary artery obstruction.
DISCUSSION
Percutaneous closure using the new Amplatzer duct occluder has significantly improved the results of tanscatheter closure of moderate sized and large ducts. Its major advantages over previous devices are the smaller delivery sheaths (6–7 French), the ability to reposition the device before release, and a significantly lower rate of complications and residual shunts.8–10 However, the Amplatzer duct occluder—as well as the other currently available duct occluders such as the Gianturco coils,4–6 the buttoned device,7 and the Gianturco-Grifka vascular occluding device14—are not designed to maintain a stable position under high pressure. Thus in the setting of high pulmonary artery pressure there is a real possibility of systemic embolisation. In fact, we know of two instances of embolisation of an Amplatzer duct occluder into the aorta (Amplatz K, personal communication). In both these cases there was a systemic, and apparently following occlusion a suprasystemic, pulmonary artery pressure (pulmonary hypertensive crisis).
The findings of our present study—which we believe describes the first clinical experience with the Amplatzer muscular ventricular septal occluder for closing persistent arterial ducts—indicate that transcatheter closure of large HPAP-PDAs is feasible, effective, and safe. Complete occlusion was obtained in all patients, with a significant fall in the pulmonary artery pressure and no complications during the procedure or at the one year follow up. The device used is a modified Amplatzer septal occluder which has all the previously described advantages of the Amplatzer duct occluder, while its retention disk system ensures secure positioning in the pulmonary orifice of the duct and prevents device embolisation into the systemic circulation in the presence of high pulmonary artery pressure. In addition, because of its construction from tightly woven Nitinol wire, the AMVSDO exerts an exaggerated stenting effect on the duct wall, giving it greater stability than the Amplatzer duct occluder. Finally, this occluding device is available in sizes up to 24 mm, which makes it suitable for transcatheter closure of very large ducts.
Oversizing of the Amplatzer duct occluder has been reported to play an important role in achieving a virtually 100% occlusion rate.9 In our study the device was selected according to the balloon stretched diameter of the duct, which was 2–3 mm larger than the angiographically estimated diameter. This not only increases the efficacy of the procedure but also reduces the risk of embolisation by achieving better device fixation and stability across the duct channel.
In this series of patients pulmonary vascular resistance was not included among the criteria for proceeding to duct closure. We believe that balloon duct occlusion is a useful test to define the reversibility of pulmonary artery hypertension in HPAP-PDA (systolic pulmonary artery pressure more than two thirds of the systemic pressure). A significant fall in pulmonary artery pressure during balloon duct occlusion indicates potentially reversible pulmonary artery hypertension. However, evaluation of the reduction in the calculated pulmonary vascular resistance in response to oxygen or drug administration is recommended in cases of HPAP-PDA with fixed or non-significantly reduced pulmonary artery pressure during the balloon occlusion test.
Study limitations
While we successfully closed large ducts ranging from 7–13 mm in size and 6–8 mm in length with an Amplatzer muscular ventricular septal defect occluder, this device was not specifically designed for this purpose and has two limitations. Firstly, it is only available in one length, and ducts vary considerably in length. With very short ducts the device will extend too far into the pulmonary artery and may cause partial obstruction. Secondly, the retention disks are at a right angle but the insertion of the duct into the aorta and pulmonary artery is angled.9 In young children, therefore, the aortic disk may extend too far into the aorta and cause significant obstruction. A more ideal design would have been a prosthesis available in several lengths with two angled disks.
Conclusions
The AMVSDO is an important adjunct for closure of large ducts associated with high pulmonary artery pressure. Further studies are required to document its efficacy, safety, and long term results in a larger number of patients.
Abbreviations
AMVSDO, Amplatzer muscular ventricular septal defect occluder
HPAP-PDA, high pulmonary artery pressure ducts
PDA, persistent ductus arteriosus
This study was presented at the World Congress of Pediatric Cardiology and Cardiac Surgery, Toronto, May 27–31, 2001.
REFERENCES
- 1.Rashkind WJ, Mullins CE, Helenbrand WE, et al. Non-surgical closure of patent ductus arteriosus: clinical application of the Rashkind PDA occluder system. Circulation 1987;75:583–92. [DOI] [PubMed] [Google Scholar]
- 2.Hosking MCK, Benson LN, Musewe N, et al. Transcatheter occlusion of the persistently patent ductus arteriosus: forty-month follow-up and prevalence of residual shunting. Circulation 1991;84:2313–17. [DOI] [PubMed] [Google Scholar]
- 3.Verin VE, Saveliev VS, Kolody SM, et al. Results of transcatheter closure of the patent ductus arteriosus with the Botalloocluder. J Am Coll Cardiol 1993;22:1509–14. [DOI] [PubMed] [Google Scholar]
- 4.Hijazi ZM, Geggel RL. Results of anterograde transcatheter closure of patent ductus arteriosus using single or multiple Gianturco coils. J Am Coll Cardiol 1994;74:925–9. [DOI] [PubMed] [Google Scholar]
- 5.Hijazi ZM, Geggel RL. Transcatheter closure of large patent ductus arteriosus (≥ 4 mm) with multiple Gianturco coils: immediate and mid-term results. Heart 1996;76:536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzun O, Hancock S, Parsons JM, et al. Transcatheter occlusion of the atrial duct with Cook detachable coil: early experience. Heart 1996;76:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao PS, Kim SH, Choi JY, et al. Follow-up of transvenous occlusion of patent ductus arteriosus with the buttoned device. J Am Coll Cardiol 1999;33:820–6. [DOI] [PubMed] [Google Scholar]
- 8.Masura J, Kevin P, Thanopoulos B, et al. Catheter closure of moderate- to large-sized patent ductus arteriosus using the new Amplatzer Duct Occluder: immediate and short-term results. J Am Coll Cardiol 1998;31:878–82. [DOI] [PubMed] [Google Scholar]
- 9.Thanopoulos B(V)D, Hakim FA, Hiari A, et al. Further experience with transcatheter closure of the patent ductus arteriosus using the Amplatzer duct occluder. J Am Coll Cardiol 2000;35:1016–21. [DOI] [PubMed] [Google Scholar]
- 10.Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the Amplatzer PDA device: immediate results of the international clinical trial. Cathet Cardiovasc Intervent 2000;51:50–4. [DOI] [PubMed] [Google Scholar]
- 11.Thanopoulos B(V)D, Tsaousis GS, Konstadopoulou GN, et al. Transcatheter closure of muscular ventricular septal defects with the Amplatzer ventricular septal defect occluder: initial clinical application in children. J Am Coll Cardiol 1999;33:1395–9. [DOI] [PubMed] [Google Scholar]
- 12.Hijazi ZM, Hakim F, Al-Fadley F, et al. Transcatheter closure of single muscular ventricular septal defects using the Amplatzer muscular VSD occluder: initial results and technical considerations. Cathet Cardiovasc Intervent 2000;49:167–72. [DOI] [PubMed] [Google Scholar]
- 13.Krichenko A, Benson LN, Burrows P, et al. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol 1989;67:877–80. [DOI] [PubMed] [Google Scholar]
- 14.Ebeid MR, Gaymes CH, Smith JC, et al. Gianturco-Grifka vascular occlusion device for closure of patent ductus arteriosus. Am J Cardiol 2001;87:657–60. [DOI] [PubMed] [Google Scholar]