Abstract
Background: Transmitral Doppler flow indices are used to evaluate diastolic function. Recently, velocities measured by Doppler tissue imaging have been used as an index of left ventricular relaxation.
Objective: To determine whether Doppler tissue velocities are influenced by alterations in preload.
Methods: Left ventricular preload was altered in 17 patients (all men, mean (SD) age, 49 (8) years) during echocardiographic measurements of left ventricular end diastolic volume, maximum left atrial area, peak early Doppler filling velocity, and left ventricular myocardial velocities during early filling. Preload altering manoeuvres included Trendelenberg (stage 1), reverse Trendelenberg (stage 2), and amyl nitrate (stage 3). Systolic blood pressure was measured at each stage.
Results: In comparison with baseline, left ventricular end diastolic volume (p = 0.001), left atrial area (p = 0.003), peak early mitral Doppler filling velocity (p = 0.01), and systolic blood pressures (p = 0.001) were all changed by preload altering manoeuvres. Only left ventricular myocardial velocity during early filling remained unchanged by these manoeuvres.
Conclusions: In contrast to standard transmitral Doppler filling indices, Doppler tissue early diastolic velocities are not significantly affected by physiological manoeuvres that alter preload. Thus Doppler tissue velocities during early left ventricular diastole may provide a better index of diastolic function in cardiac patients by providing a preload independent assessment of left ventricular filling.
Keywords: Doppler tissue echocardiography, preload alterations
Diastolic dysfunction is the primary mechanism responsible for dyspnoea in patients with heart failure, irrespective of the presence or severity of systolic dysfunction.1–3 Left ventricular diastolic dysfunction usually precedes systolic dysfunction, and abnormal relaxation is observed at its earliest stage.2–6 Conventional clinical evaluation of left ventricular relaxation involves determining the time constant of pressure decay during isovolumic diastole, as calculated from the left ventricular pressure curve.7
Doppler echocardiography has become the non-invasive technique of choice for evaluating diastolic function.8,9 Pulmonary venous Doppler flow indices have been used to evaluate different indices of diastolic function, including left ventricular filling, pressure, relaxation, and stiffness.8,10–12 Unfortunately, as several physiological variables—including volume status, left atrial pressure, and the rate of myocardial relaxation13–15—affect Doppler flow velocities simultaneously, it is often difficult to determine which individual variables are responsible when a specific Doppler pattern is observed, unless other relevant clinical information is available.6,16–18
Blood flow Doppler tissue imaging (DTI) is a new ultrasound method that records systolic and diastolic velocities within the myocardium19–23 and at the corner of the mitral annulus.24–26 The early diastolic tissue velocity (EDTV) recorded at the lateral corner of the annulus has been shown recently to decline progressively with age and to be reduced in pathological left ventricular hypertrophy25 and in patients with restrictive cardiomyopathy.26 These findings suggest that EDTV is an index of left ventricular relaxation that may not be influenced by left atrial pressure.
Our aim in the present investigation was therefore to assess whether the EDTV as recorded by Doppler tissue imaging is a preload independent index of left ventricular relaxation in patients with a pseudonormalised mitral inflow pattern.
METHODS
The study group consisted of 17 patients (all men, mean (SD) age, 49 (8) years) with a stable form of chronic ischaemic syndrome. Each patient underwent echocardiographic evaluation in our laboratory for assessment of cardiac structure and function. Patient selection was according to diastolic function, with all the patients having a pseudonormal left ventricular diastolic filling pattern. End diastolic volumes were very different because there was a large geometric variation in left ventricular cavity size in the patients. All patients were in sinus rhythm. Exclusion criteria were congestive heart failure, valvar heart disease, primary myocardial heart disease, secondary hypertrophy (hypertension, aortic stenosis, and so on), and endocrinological and renal diseases. All subjects gave written informed consent before participation.
Echocardiographic studies
Echocardiography was performed using an Acuson 128 × P10 (Acuson Mountain View, California, USA) equipped with a variable frequency, phased array transducer (2.5–4.0 MHz) with DTI capabilities.
Images were taken in the left lateral decubitus position and the complete echocardiographic study was performed using standard views and techniques. Cross sectional studies were recorded from the parasternal long and short axis and the apical four and two chamber views. End diastolic volumes and end systolic left atrial area were obtained from the apical four chamber view. All Doppler echocardiographic and tissue imaging recordings were obtained during normal respiration.
Pulsed Doppler echocardiography
The sample volume was set at the mitral valve orifice in the long axis view of the left ventricle or the four chamber view recorded from the cardiac apex, and transmitral flow velocity patterns were recorded. Early diastolic wave velocity was then obtained.
Doppler tissue imaging
In the parasternal long axis view of the left ventricle, sample volumes were set at the endocardial portions of the basal, middle, and apical sites of both left ventricular walls, and mean values were determined (that is, for ventricular septum and posterior wall). Because there were variable left ventricular geometric abnormalities resulting from ischaemic heart disease or systolic dysfunction in these patients, myocardial tissue was the preferred source of tissue Doppler measurements to assess the effect of preload changes on the ischaemic tissue. The left ventricular parasternal long axis was used to provide the optimal angle during the Doppler tissue imaging measurements. We believe this reduces the likelihood of global translation effects but does not totally eliminate them, and this remains a limitation of the study. The motion velocity patterns at each site were recorded by the pulsed Doppler method. The EDTV was then determined from the patterns obtained. After all the baseline indices had been obtained, the studies were repeated during preload altering manoeuvres. These various stages were as follows: baseline; stage 1 (Trendelenberg position); stage 2 (reverse Trendelenberg position); stage 3 (amyl nitrate inhalation). At all stages systolic blood pressure was measured continuously.
Statistical analysis
Data are presented as mean (SD). Analysis of variance and t testing were used to compare differences between the stages. A probability value of p < 0.05 was considered significant.
RESULTS
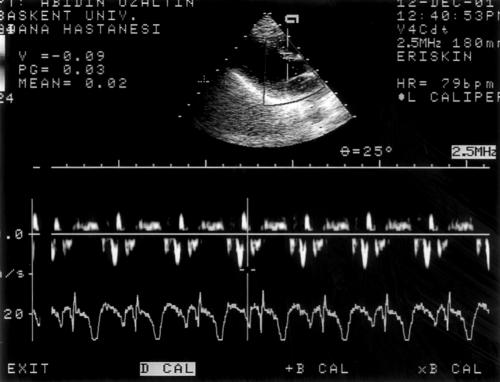
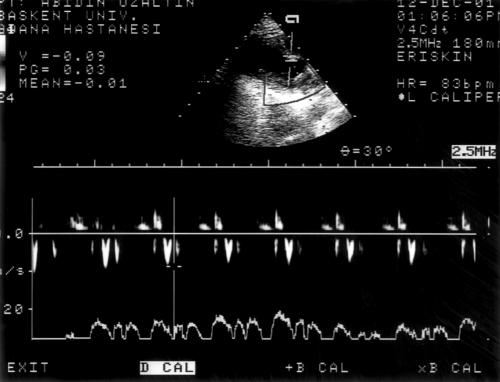
We report results from 17 consecutive patients with systolic and diastolic dysfunction caused by coronary artery disease. The mean ejection fraction was 32 (13)%, 35 (11)%, 34 (11)%, and 33 (14)% during stages 1 to 4, respectively. Baseline left ventricular end diastolic volume (LVEDV), left atrial area, left ventricular early mitral inflow velocity (E), and EDTV were obtained in all patients (figs 1 and 2).
Figure 1.
Measurement of basal myocardial tissue Doppler early diastolic filling velocity in a patient with pseudonormal filling pattern.
Figure 2.
After trendelenberg manoeuvre, repeated measurement of early diastolic filling velocity in the same patient.
In stage 1, early diastolic mitral inflow velocity profile increased (p = 0.01) with the Trendelenberg manoeuvre, which is known to increase the preload. In that stage, LVEDV (p = 0.001) and left atrial area (p = 0.003) increased significantly, but EDTV did not change (p < 0.05). With preload decreasing manoeuvres such as the reverse Trendelenberg position and amyl nitrate inhalation, EDTV also remained unchanged, though the other variables changed significantly (table 1).
Table 1.
Changes in echocardiographic measurements during preload altering manoeuvres
| Variable | Baseline | Stage 1 | Stage 2 | Stage 3 |
| LVEDV (ml)* | 198.6 (132.2) | 242.2 (186.2) | 212.1 (163.0) | 188.1 (132.9) |
| Left atrial area (mm2)† | 22.4 (8.6) | 23.9 (10.0) | 21.7 (9.6) | 20.1 (7.7) |
| E wave (cm/s)‡ | 82.4 (33.1) | 84.6 (32.2) | 71.9 (25.9) | 72.8 (20.2) |
| EDTV (cm/s)§ | 10.3 (3.5) | 10.7 (3.1) | 10.7 (3.4) | 11.1 (3.7) |
| Systolic blood pressure (mm Hg)* | 126.4 (18.6) | 122.9 (16.9) | 118.1 (21.0) | 103.2 (21.5) |
Values are mean (SD).
p Values: stages 1–3 v baseline: *p=0.001; †p=0.003; ‡p=0.01; §p=NS (p>0.05).
Stage 1, Trendelenberg manoeuvre; stage 2, reverse Trendelenberg manoeuvre; stage 3, amyl nitrate inhalation.
E, left ventricular early mitral inflow velocity; EDTV, early diastolic velocity; LVEDV, left ventricular end diastolic volume.
DISCUSSION
The results of our study suggest that peak Doppler tissue early left ventricular velocities are not affected by varying preload conditions. EDTV did not change significantly in spite of the changes in haemodynamic variables.
Mitral inflow variables are load dependent, and patients with a relaxation abnormality may show a normal pattern with a raised atrial pressure. This can occur because mitral inflow variables are velocity determined, reflecting the pressure difference between the left atrium and the left ventricle during diastole. The assessment of volume change has the theoretical advantage of being less preload dependent than mitral inflow variables. Garcia and colleagues observed that peak EDTV correlated poorly with peak E velocity, suggesting the relative preload independence of peak EDTV.26
In this study we showed that, in contrast to mitral inflow velocity, peak early tissue velocity did not change significantly after alteration of the preload by the Trendelenberg manoeuvre, the reverse Trendelenberg manoeuvre, and inhalation of amyl nitrate. This result may reflect the decreased kinetics of ischaemic myocardial tissue, which is not significantly affected by preload change during early diastole. Sohn and colleagues obtained similar results by altering the preload with an infusion of saline or glyceryl trinitrate.27 In heart transplant cases, Aranda and associates also showed that if preload was altered by glyceryl trinitrate during routine examination, there was no change in peak EDTV.28
In contrast to standard Doppler echocardiography, Doppler tissue imaging can measure myocardial tissue velocity, which directly reflects contractile and relaxation properties of the myocardium. All our subjects had evidence of decreased left ventricular systolic function and coronary artery disease, as detected by coronary angiography. There was no pericardial effusion. The only factors that were altered by the changes in preload and afterload were systolic blood pressure (p = 0.001), LVEDV (p = 0.001), and left atrial area (p = 0.003). Changes in the haemodynamic profile after inhalation of amyl nitrite—like the increase in heart rate and the decrease in blood pressure, LVEDV and left atrial area—are well known and are probably mediated by venous and arterial dilatation through reflex sympathetic stimulation by baroreceptors.29 The relaxation velocities at different preload conditions did not change significantly, suggesting that myocardial relaxation is independent of preload. This is consistent with the findings of Stoddard and colleagues.30
Impaired relaxation is common to all patients with heart failure,1,3 the left atrial pressure increasing in response to a reduction in left ventricular compliance in this condition. This increase masks the influence of impaired relaxation on the transmitral velocity and produces a pseudonormal pattern with an E/A ratio > 1 and a shortening of the isovolumic relaxation and deceleration times.9,10,31 These patients, however, continue to show abnormal myocardial relaxation, which can be demonstrated by invasive measurements of the time constant of relaxation10 and more recently by the flow propagation velocity of the left ventricular inflow, assessed by colour M mode echocardiography.32–34
In the current study, EDTV provided a preload independent assessment of left ventricular filling, establishing it as a useful index of diastolic function in patients with known cardiac disease. However, in previous work from our group, it was shown that EDTV is preload dependent in normal animals35 and in humans without cardiac disease.36 Interestingly, in the animal study, this preload dependence was blunted when ventricular relaxation was delayed by β adrenergic blockade. As delayed relaxation is one of the earliest manifestations of a variety of cardiac diseases, in the vast majority of cardiac patients EDTV should be relatively independent of preload. However, in patients with normal hearts but reduced stroke volume—as in hypovolaemia, for example—EDTV is also likely to be depressed. The importance of EDTV as a preload independent index of left ventricular relaxation goes beyond the simple distinction of the pseudonormal mitral inflow pattern from normal. In most patients this distinction can often be deduced from clinical and echocardiographic variables that suggest the presence of impaired relaxation, and by inspection of pulmonary vein velocity. Recently greater importance has been placed on the possibility that EDTV could be used as a variable independent of preload for detecting abnormalities of left ventricular relaxation.
Conclusions
In contrast to standard diastolic transmitral Doppler filling indices, Doppler tissue early diastolic velocities are not significantly affected by physiological preload altering manoeuvres. Thus EDTV during early left ventricular diastole may be a more useful index of diastolic function as it provides an afterload independent assessment of left ventricular filling.
Acknowledgments
This study was supported by National Aeronautics Space Administration grant No NCC9-60, Houston, Texas, USA, and was presented at the meeting of the American Society of Echocardiography in June 1999 in Washington DC.
Abbreviations
DTI, Doppler tissue imaging
E, left ventricular early mitral inflow velocity, EDTV, early diastolic tissue velocity
LVEDV, left ventricular end diastolic volume
REFERENCES
- 1.Grossman W, McLaurin LP, Rolett EL. Alterations in left ventricular relaxation and diastolic compliance in congestive cardiomyopathy. Cardiovasc Res 1979;13:514–22. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty AH, Nacarelli GV, Gray EL, et al. Congestive heart failure with normal systolic function. Am J Cardiol 1984;54:778–82. [DOI] [PubMed] [Google Scholar]
- 3.Grossman W. Diastolic dysfunction and congestive heart failure. Circulation 1990;81(suppl III):III-1–7. [PubMed] [Google Scholar]
- 4.Grossman W, McLaurin LP. Diastolic properties of the left ventricle. Ann Intern Med 1976;84:316–26. [DOI] [PubMed] [Google Scholar]
- 5.Bonow RO, Bacharach SL, Green MV, et al. Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation 1981;64:315–23. [DOI] [PubMed] [Google Scholar]
- 6.Ishiada Y, Meisner JS, Tsujioka K, et al. Left ventricular filling dynamics: influence of left ventricular relaxation and left atrial pressure. Circulation 1986;74:187–96. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JL, Fredriksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 1976;58:751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura RA, Abel MD, Hatle LK, et al. Assessment of diastolic function of the heart: background and current applications of Doppler echocardiography. Part II. Clinical studies. Mayo Clin Proc 1989;64:181–204. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JD, Weyman AE. Echo Doppler evaluation of left ventricular diastolic function: physics and physiology, Circulation 1991;84:990–7. [DOI] [PubMed] [Google Scholar]
- 10.Appleton CP, Hatle LK, Pop RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988;12:426–40. [DOI] [PubMed] [Google Scholar]
- 11.Hoit BD, Walsh RA. Diastolic function in hypertensive heart disease. In: Gaasch WH, Le Winter M, eds. Left ventricular diastolic dysfunction and heart failure. Philadelphia: Lea and Febiger 1994:354–72.
- 12.Labowitz AJ, Pearson AC. Evaluation of left ventricular diastolic function: clinical relevance and recent Doppler echocardiographic insights. Am Heart J 1987;114:836–51. [DOI] [PubMed] [Google Scholar]
- 13.Choong CY, Hermann HC, Weyman AE, et al. Preload dependence of Doppler derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol 1987;10:800–8. [DOI] [PubMed] [Google Scholar]
- 14.Gardin JM, Rohan MK, Davidson DM. Doppler transmitral flow velocity parameters: relation between age, body surface area, blood pressure and gender in normal subjects. Am J Noninvasive Cardiol 1987;1:3–10. [Google Scholar]
- 15.Kuo LC, Quinones MA, Rokey R, et al. Quantification of atrial contribution to left ventricular filling by pulsed Doppler echocardiography and the effect of age in normal and diseased hearts. Am J Cardiol 1987;59:1174–8. [DOI] [PubMed] [Google Scholar]
- 16.Appleton CP, Hatle LK. The natural history of left ventricular filling abnormalities: assessment by two dimensional and Doppler echocardiography. Echocardiography 1992;9:437–45. [Google Scholar]
- 17.Choong CY, Abascal VM, Thomas JD, et al. Combined influence of ventricular loading and relaxation on the transmitral flow velocity profile in dogs measured by Doppler echocardiography. Circulation 1988;78:672–83. [DOI] [PubMed] [Google Scholar]
- 18.Colan SD, Borrow KM, Neumann A. Effects of loading conditions and contractile state (methoxamine and dobutamine) on left ventricular early diastolic function in normal subjects. Am J Cardiol 1985;55:790–6. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland GR, Stewart MJ, Groundstroem KWE, et al. Color Doppler myocardial imaging: a new technique for the assessment of myocardial function . J Am Soc Echocardiogr 1994;7:441–58. [DOI] [PubMed] [Google Scholar]
- 20.Miyatake K, Yamagishi M, Tanaka N, et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J Am Coll Cardiol 1995;25:717–24. [DOI] [PubMed] [Google Scholar]
- 21.Donovan CL, Armstrong WF, Bach DS. Quantitative Doppler tissue imaging of the left ventricular myocardium: validation in normal subjects. Am Heart J 1995;130:100–4. [DOI] [PubMed] [Google Scholar]
- 22.Uematsu M, Miyatake K, Tanaka N, et al. Myocardial velocity gradient as a new indicator of regional left ventricular contraction: detection by a two dimensional tissue Doppler imaging technique. J Am Cardiol 1995;26:217–23. [DOI] [PubMed] [Google Scholar]
- 23.Groscan J, Gulati VK, Mandarino WA, et al. Color coded measures of myocardial velocity throughout the cardiac cycle by tissue Doppler imaging to quantify regional left ventricular function. Am Heart J 1996;131:1202–13. [DOI] [PubMed] [Google Scholar]
- 24.Isaaz K, Munoz del Romeral L, et al. Quantification of the motion of the cardiac base in normal subjects by Doppler echocardiography. J Am Soc Echocardiogr 1993;6:166–76. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez L, Garcia MG, Ares M, et al. Assessment of mitral annular dynamics during diastole by Doppler tissue imaging: comparison with mitral Doppler inflow in subjects without heart disease and in patients with left ventricular hypertrophy. Am Heart J 1996;131:982–7. [DOI] [PubMed] [Google Scholar]
- 26.Garcia MG, Rodriguez L, Ares M, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy: assessment of left ventricular diastolic velocities in longitudinal axis by Doppler tissue imaging. J Am Coll Cardiol 1996;27:108–14. [DOI] [PubMed] [Google Scholar]
- 27.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–80. [DOI] [PubMed] [Google Scholar]
- 28.Aranda JM, Weston MW, Puleo JA, et al. Effect of loading conditions on myocardial relaxation velocities determined by Doppler tissue imaging in heart transplant recipients. J Heart Lung Transplant 1998;17:693–7. [PubMed] [Google Scholar]
- 29.Lundbrook P, Byrne J, Jurnik R, et al. Influence of reduction of preload and afterload by nitroglycerin on left ventricular diastolic pressure–volume relations and relaxation in man. Circulation 1977;56:937–43. [DOI] [PubMed] [Google Scholar]
- 30.Stoddard M, Pearson A, Kern M, et al. Influence of alteration in preload on the pattern of left ventricular diastolic filling as assessed by Doppler echocardiography in humans. Circulation 1989;79:1226–36. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JD, Choong CYP, Flachskampf FA, et al. Analysis of the early transmitral Doppler velocity curve: effects of primary physiologic changes and compensatory preload adjustment. J Am Coll Cardiol 1990;16:644–55. [DOI] [PubMed] [Google Scholar]
- 32.Brun P, Tribwilly C, Duval AM. Left ventricular flow propagation during early filling is related to wall relaxation: a colour M-mode Doppler analysis. J Am Coll Cardiol 1992;20:420–32. [DOI] [PubMed] [Google Scholar]
- 33.Stügaard M, Smseth OA, Risoe C, et al. Intraventricular early diastolic filling during acute myocardial ischemia; assessment by multigated color M-mode Doppler echocardiography. Circulation 1993;88:2705–13. [DOI] [PubMed] [Google Scholar]
- 34.Takatsuji H, Mikami T, Urasawa K, et al. A new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol 1996;27:365–71. [DOI] [PubMed] [Google Scholar]
- 35.Firstenberg MS, Greenberg NL, Main ML, et al. Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload. J Appl Physiol 2001;90:299–307. [DOI] [PubMed] [Google Scholar]
- 36.Firstenberg MS, Levine BD, Garcia MJ, et al. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol 2000;36:1664–9. [DOI] [PubMed] [Google Scholar]