Abstract
Objective: To assess whether diastolic graft function is influenced by intragraft interleukin 2 (IL-2) messenger RNA (mRNA) expression in rejecting cardiac allografts.
Design: 16 recipients of cardiac allografts were monitored during the first three months after transplantation. The presence of IL-2 mRNA in endomyocardial biopsies (n = 123) was measured by reverse transcriptase polymerase chain reaction. To determine heart function, concurrent M mode and two dimensional Doppler echocardiograms were analysed.
Results: Histological signs of acute rejection (International Society for Heart and Lung Transplantation (ISHLT) rejection grade > 2) were strongly associated with IL-2 mRNA expression (IL-2 mRNA was present in 12 of 20 endomyocardial biopsies (60%) with acute rejection and in 24 of 103 endomyocardial biopsies (23%) without acute rejection, p = 0.002). No significant relation was found between either histology or IL-2 mRNA expression alone and the studied echocardiographic parameters. However, stratification of the echocardiographic data into those of patients with and those without acute rejection showed that during acute rejection IL-2 mRNA expression was significantly associated with increased left ventricular total wall thickness (mean change in total wall thickness was +0.22 cm in patients with IL-2 mRNA expression versus −0.18 cm in patients without IL-2 mRNA expression, p = 0.048).
Conclusions: An increase in left ventricular total wall thickness precedes IL-2 positive acute rejection after heart transplantation. Thus, cardiac allograft rejection accompanied by intragraft IL-2 mRNA expression may be indicative of more severe rejection episodes.
Keywords: transplantation, echocardiography, acute rejection, interleukin-2
Reversible diastolic dysfunction of cardiac allografts in the first postoperative months has been reported to correlate with reduced long term survival.1 This diastolic dysfunction can be the result of acute rejection, a major complication shortly after transplantation.2 However, not all histologically proven acute rejection episodes result in changes of diastolic function parameters. The underlying mechanism of the difference between acute rejection episodes with and those without impaired heart function is unknown but may involve the local or systemic release of cytokines.2
Acute cellular rejection is morphologically characterised by the presence of a mononuclear cell infiltrate and signs of myocyte damage on endomyocardial biopsy (EMB).3 The combination of infiltrated immune competent cells with interstitial and perivascular oedema, vascular leakage of fibrin, and in the more severe cases diffuse myocyte necrosis can result in stiffness of the myocardium.4 Consequently, the acute rejection process may change left ventricular wall dimensions and left ventricular filling parameters, reflecting the loss of diastolic heart function. Previously reported echocardiographic changes related to acute cellular rejection include increased left ventricular total wall thickness, increased peak early mitral flow velocity (E), decreased deceleration time, and shortened isovolumetric relaxation period.5,6 Alternatively, during vascular (humoral) rejection, cells of the microvasculature instead of myocytes may be the target of tissue injury, which has been associated with increased left ventricular mass and depressed myocyte function.4,7
In addition to cell infiltration and tissue damage, local production of cytokines (low molecular weight regulatory proteins) during rejection may also influence myocardial function.8,9 One of the crucial cytokines in the modulation of the alloimmune response is interleukin (IL) 2, which enhances the proliferation and differentiation of specific T lymphocytes directed against the allograft.10,11 In non-transplant settings, IL-2 has been shown to cause dysfunction of the heart both in experimental and in clinical studies.12,13 A dose dependent negative inotropic effect of IL-2 was observed in isolated hamster papillary muscle preparations.12 Moreover, intravenous administration of IL-2 in patients with cancer causes cardiovascular side effects including decreased ejection fraction, decreased blood pressure, increased heart rate, and fluid retention.13 Therefore, we speculate that after transplantation the local presence of IL-2 may be related to allograft dysfunction by reflecting the severity of the immune response against the allograft and by its direct negative inotropic effect on the myocardium. To determine whether local IL-2 expression during histological rejection indeed affects diastolic graft function, we monitored serially sampled EMB for the presence of IL-2 messenger RNA (mRNA) expression and for histological signs of acute rejection during the first three months after heart transplantation. These findings were analysed in relation to echocardiographic changes in wall dimensions and diastolic flow parameters.
METHODS
Patients
We studied 16 consecutive heart transplant recipients who were operated upon between November 1997 and October 1998. Table 1 summarises the demographic data of these patients. Maintenance immunosuppressive therapy consisted of ciclosporin A and low dose steroids. Serial EMB and echocardiograms during the first three months after transplantation were studied. Surveillance biopsies during this period were taken weekly during the first six weeks and biweekly during the following eight weeks. During routine biopsies two additional samples were harvested for cytokine studies after informed consent of the patients. One patient has been excluded from analysis because of the poor quality of serial echocardiograms, leaving 15 patients in the study group. In total, 123 biopsies with concurrent echocardiograms (on average eight time points per patient) were available for analysis. Acute rejection was diagnosed by histological assessment of myocardial biopsies and graded according to the guidelines of the International Society for Heart and Lung Transplantation (ISHLT).3 Patients with ISHLT rejection grade > 2 were considered to have an acute rejection (AR+) and received additional immunosuppressive treatment.
Table 1.
Demographics of the studied patients and the numbers of endomyocardial biopsies (EMB) positive for acute rejection (AR+) and interleukin 2 (IL-2) messenger RNA (mRNA) expression (IL-2+)
| Patient | Age (years) | Sex | Primary disease | EMB | AR+ EMB | IL-2+ EMB | AR+/IL-2+ | AR−/IL-2+ |
| 1 | 20 | F | CMP | 8 | 2 | 1 | 1 | 0 |
| 2 | 52 | M | IHD | 9 | 0 | 5 | 0 | 5 |
| 3 | 60 | M | IHD | Excluded from the study due to poor echocardiographic quality | ||||
| 4 | 48 | M | IHD | 9 | 2 | 4 | 2 | 2 |
| 5 | 58 | M | CMP | 8 | 1 | 1 | 1 | 0 |
| 6 | 51 | M | CMP | 8 | 1 | 2 | 1 | 1 |
| 7 | 56 | F | CMP | 7 | 1 | 3 | 1 | 2 |
| 8 | 58 | M | IHD | 9 | 2 | 3 | 2 | 1 |
| 9 | 53 | F | CMP | 8 | 4 | 2 | 0 | 2 |
| 10 | 50 | M | IHD | 7 | 1 | 1 | 0 | 1 |
| 11 | 16 | F | CMP | 6 | 2 | 2 | 2 | 0 |
| 12 | 54 | M | IHD | 8 | 2 | 4 | 1 | 3 |
| 13 | 43 | M | CMP | 8 | 2 | 3 | 1 | 2 |
| 14 | 55 | M | IHD | 9 | 0 | 2 | 0 | 2 |
| 15 | 58 | M | IHD | 9 | 0 | 2 | 0 | 2 |
| 16 | 52 | M | IHD | 10 | 0 | 1 | 0 | 1 |
| Total | 123 | 20 | 36 | 12 | 24 | |||
CMP, cardiomyopathy; F, female; IHD, ischaemic heart disease; M, male.
Echocardiography
Echocardiographic examination was completed within four hours of biopsy sampling using a Hewlett Packard Sonos 500 or 5500 ultrasonograph (Hewlett Packard, Palo Alta, California, USA) with a 3.75 MHz transducer. All recordings were made and analysed by a single investigator without knowledge of the results of EMB. M mode recordings for measurement of left ventricular wall dimensions were obtained from the parasternal long axis view in combination with an ECG, phonocardiogram, and respiratory tracing. For two dimensional Doppler echocardiography the transducer was positioned at the cardiac apex for a standard apical four chamber view. Mitral flow velocities were recorded within the valve orifice near the leaflet tips with the sample volume placed parallel to the ventricular inflow tract.
Offline, M mode, and two dimensional Doppler echocardiograms were analysed using a software program developed in our laboratory with a hand held digitiser connected to a digitising tablet (Summa Sketch Plus, Summagraphics, Seymour, Connecticut, USA) interfaced with a personal computer. For M mode echocardiogram analysis, means of five consecutive end expiratory beats were calculated. M mode echocardiograms were analysed for end diastolic posterior left ventricular wall thickness and end diastolic interventricular septum thickness. Left ventricular total wall thickness was calculated by adding end diastolic posterior wall thickness and end diastolic interventricular septum thickness. Two dimensional Doppler echocardiograms were analysed for the following diastolic function parameters: E, peak atrial mitral flow velocity (A), E/A ratio, deceleration time of E, and isovolumetric relaxation period. For Doppler echocardiogram analysis, means of 10 consecutive end expiratory beats were calculated. Beats that were distorted by the recipient atrial contraction were not analysed with respect to deceleration time. The transmitral filling pattern was classified as “summation filling” when only one peak occurred in diastole after the P wave of the succeeding beat. In such recordings, E and A waves could not be recognised and consequently the parameters E, A, E/A ratio, and deceleration time were not measured.
IL-2 mRNA detection
Reverse transcriptase polymerase chain reaction (PCR) was used for detection of IL-2 mRNA expression. Therefore, total RNA was extracted from snap frozen EMB, and subsequently complementary DNA (cDNA) was synthesised with random primers as described previously in detail.14 Aliquots of cDNA (representing 1/20 EMB) were directly used for PCR amplification using sequence specific primers for IL-2 (sense: 5`-ATG TAC AGG ATG CAA CTC CTG TCT T-3`; antisense: 5`-GTC AGT GTT GAG ATG ATG CTT TGA C-3`). Sample cDNA (5 μl) was added to a 95 μl PCR mixture containing 10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each of deoxy-ATP, deoxy-CTP, deoxy-TTP, and deoxy-GTP, 2 U Taq polymerase (Applied Biosystems, Norwalk, Connecticut, USA) and overlaid with 100 μl mineral oil (Sigma, St Louis, Missouri, USA) before PCR in a DNA thermal cycler model 480 (Applied Biosystems) under the following conditions. After a 5 minute 94°C denaturation step, samples underwent 40 cycles of denaturation for 1 minute at 94°C, 2 minutes' annealing at 60°C, and 3 minutes' extension at 72°C. The last cycle was extended to 7 minutes at 72°C.
Positive control samples were produced by mRNA extraction and cDNA synthesis from 106 random human spleen cells stimulated with 1% phytohaemagglutin M (Difco, Detroit, Michigan, USA) for 24 hours at 37°C. Negative control samples consisted of diethyl pyrocarbonate treated H2O as a no template reaction. In all samples, constitutively expressed glyceraldehyde 3-phosphate dehydrogenase mRNA was amplified in a separate PCR (3-phosphate dehydrogenase primers, sense: 5`-GGT GAA GGT CGG AGT CAA CG-3`; antisense: 5`-CAA AGT TGT CAT GGA TGA CC-3`) to confirm successful mRNA extraction and cDNA transcription. EMB that were negative for 3-phosphate dehydrogenase mRNA expression were excluded from further analysis.
The presence of IL-2 mRNA on an agarose gel was visually assessed as present (IL-2 positive) or not present (IL-2 negative) and confirmed by Southern blot hybridisation. Therefore, products on the agarose gel were transferred to a Hybond-N+ membrane (Amersham, Buckinghamshire, UK) by electroblotting and subsequently hybridised with a γ32P labelled specific probe for IL-2 (5`-TTC TTC TAG ACA CTG AAG ATG TTT CAG TTC-3`), which is located across the splice site. Hybridisation was detected by autoradiography and indicated the presence of IL-2 mRNA expression in the original biopsy.
Statistical analysis
Differences in mean echocardiographic parameters between EMB with and those without either histologically proven acute rejection or IL-2 mRNA expression were analysed by Student's t test. Associations between IL-2 mRNA expression and acute rejection, as well as changes in wall dimension between the first measurement and the first acute rejection biopsy with or without IL-2 mRNA expression, were calculated by Fisher's exact test in a cross table. Associations with p < 0.05 were considered significant.
RESULTS
Acute rejection
In 4 of 16 patients no histological signs of acute rejection were observed during the first three months after transplantation. These non-rejectors served as the control group. One of the rejectors (patients with at least one AR+ biopsy) was excluded from analysis because of poor echocardiographic quality. Overall, 20 of 123 EMB were positive for acute rejection (16%). Table 1 shows the total number of AR+ biopsies with acute rejection during follow up of the patients.
IL-2 mRNA expression in relation to rejection
Serial measurements of IL-2 mRNA expression showed that every patient in the rejector group and in the non-rejector-group had at least one IL-2 positive EMB during the first three months after transplantation. Overall, IL-2 mRNA expression was detected in 36 of 123 EMB (29%). Table 1 summarises the number of IL-2 positive EMB in the presence (AR+) or absence of acute rejection (AR−) during follow up. IL-2 mRNA expression was strongly associated with histological signs of acute rejection requiring treatment. IL-2 mRNA was present in 12 of 20 EMB (60%) with histological signs of acute rejection and in 24 of 103 EMB (23%) without these histological characteristics (p = 0.002, Fisher's exact test; table 2).
Table 2.
Cross table of the number of biopsies negative and positive for acute rejection (AR−; AR+) and IL-2 mRNA expression (IL-2−; IL-2+)
| AR− | AR+ | Total | |
| IL-2− | 79 | 8 | 87 |
| IL-2+ | 24 | 12 | 36 |
| Total | 103 | 20 | 123 |
Diastolic function in relation to rejection and IL-2 mRNA expression
A large individual variation was observed in the echocardiographic measurements. No differences in the echocardiographic variables were observed at the group level between AR+ and AR− biopsies (table 3), between biopsies with or without IL-2 mRNA expression (table 3), or between the subdivided AR+ and AR− samples in biopsies with or without IL-2 mRNA expression (table 4).
Table 3.
Echocardiographic parameters at the time of EMB with (AR+) and without (AR−) acute rejection, and with (IL-2+) and without (IL-2−) IL-2 mRNA expression
| AR− | AR+ | p Value | IL-2− | IL-2+ | p Value | |
| TWT (cm) | 2.12 (0.30) | 2.12 (0.26) | 1.00 | 2.15 (0.30) | 2.12 (0.34) | 0.65 |
| E (m/s) | 0.82 (0.22) | 0.81 (0.23) | 0.85 | 0.81 (0.20) | 0.85 (0.24) | 0.35 |
| E/A ratio | 2.20 (0.70) | 2.00 (0.78) | 0.32 | 2.16 (0.74) | 2.08 (0.61) | 0.63 |
| DET (ms) | 161.9 (36.6) | 151.3 (29.8) | 0.28 | 159.6 (30.2) | 160.6 (45.0) | 0.90 |
| IRP (ms) | 64.83 (19.92) | 69.4 (20.29) | 0.41 | 64.41 (21.14) | 66.73 (20.20) | 0.63 |
Data are mean (SD).
A, peak atrial mitral flow velocity; DET, deceleration time of peak early mitral flow velocity; E, peak early mitral flow velocity; IRP,: isovolumetric relaxation period; TWT, left ventricular total wall thickness.
Table 4.
Echocardiographic parameters at the time of EMB with (AR+) and without (AR−) acute rejection and with (IL-2+) and without (IL-2−) IL-2 mRNA expression
| AR−/IL-2− | AR−/IL-2+ | p Value | AR+/IL-2− | AR+/IL-2+ | p Value | |
| TWT( cm) | 2.16 (0.30) | 2.05 (0.30) | 0.13 | 2.02 (0.14) | 2.19 (0.31) | 0.12 |
| E (m/s) | 0.82 (0.19) | 0.84 (0.28) | 0.66 | 0.71 (0.28) | 0.87 (0.17) | 0.13 |
| E/A ratio | 2.22 (0.73) | 2.14 (0.63) | 0.68 | 1.70 (0.54) | 2.18 (0.86) | 0.25 |
| DET (ms) | 161.5 (31.3) | 175.2 (30.4) | 0.11 | 143.3 (6.5) | 156.1 (37.3) | 0.32 |
| IRP (ms) | 64.69 (19.50) | 65.22 (21.60) | 0.92 | 64.64 (26.16) | 74.17 (12.07) | 0.37 |
Data are mean (SD).
A, peak atrial mitral flow velocity; DET, deceleration time of peak early mitral flow velocity; E, peak early mitral flow velocity; IRP,: isovolumetric relaxation period; TWT, left ventricular total wall thickness.
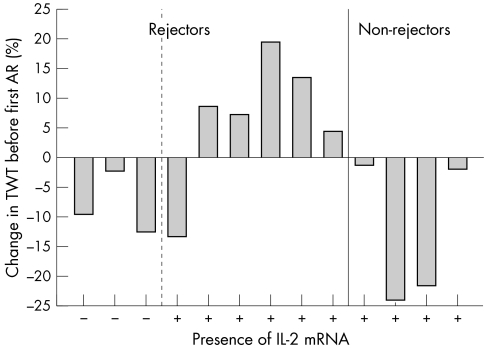
In the search for changes over time, differences in the echocardiographic parameters were calculated between the first postoperative measurement and the time of the first acute rejection or IL-2 mRNA expression. In this way, each patient served as his or her own control. Because the first echocardiographic measurement was used as the baseline, patients with acute rejection or IL-2 mRNA expression at the time of their first measurement (n = 2 and n = 4, respectively) were excluded from this specific analysis. Neither histological signs of acute rejection nor IL-2 mRNA expression alone was associated with individual changes over time in any of the studied echocardiographic parameters. However, by relating intragraft IL-2 positivity to echocardiographic changes over time after stratifying the data into AR+ and AR− time points, we found that IL-2 mRNA expression during the first acute rejection was significantly associated with increased left ventricular total wall thickness between the first postoperative measurement and the first acute rejection episode (fig 1). In five of six patients with IL-2 mRNA expression during their first acute rejection, increased total wall thickness had preceded that rejection episode (mean (SD) change between the first postoperative measurement and the measurement during the first acute rejection episode was +0.22 (0.12) cm). In all patients without IL-2 mRNA expression during their first acute rejection, total wall thickness decreased (mean (SD) −0.18 (0.12) cm; p = 0.048, Fisher's exact test; fig 1). In all non-rejecting control patients, total wall thickness decreased during their first IL-2 positive EMB with respect to the first postoperative measurement (mean (SD) −0.30 (0.31) cm). The only patient in whom total wall thickness had decreased preceding an IL-2 positive acute rejection episode had an exceptionally high total wall thickness of 2.68 cm during the first postoperative measurement, which may be attributable to the known history of hypertension of the donor. During the IL-2 positive rejection episode, the total wall thickness of this patient decreased to 2.32 cm, which is still relatively high.
Figure 1.
Change in left ventricular total wall thickness (TWT) (%) from the first postoperative measurement to the first acute rejection (AR) in patients without (n = 3) and with (n = 6) interleukin 2 (IL-2) messenger RNA expression during that first acute rejection (left) and to the first IL-2 positive biopsy in control patients with no signs of acute rejection episode (n = 4, right); p = 0.048, Fisher's exact test.
DISCUSSION
Diastolic function in relation to rejection
Numerous investigators have explored the clinical use of echocardiography to replace invasive EMB sampling for the diagnosis of acute rejection.2,5,6,15,16 During acute rejection, high left atrial pressures combined with increased myocardial stiffness can lead to a number of echocardiographic changes such as early opening of the mitral valve (short isovolumetric relaxation period); rapid filling of the left ventricle during the passive filling phase in association with a rapid increase of left ventricular pressure (high E and decreased deceleration time); and subsequent small contribution of atrial contraction to left ventricular filling (low A and increased E/A ratio).4 Although some authors described an association between acute rejection and echocardiographic variables at the group level, the considerable overlap between individual patients still limits replacement of EMB as the benchmark for diagnosis of acute rejection after heart transplantation.
In the present study, no direct association was found at the group level between the studied echocardiographic parameters and histological evidence of acute rejection alone. This is in agreement with earlier findings and can be explained by the influence of numerous factors. Besides the risk of false negative biopsies and the effect of the immune response, left ventricular filling properties and heart morphology can be affected by loading conditions, hypertension, oedema resulting from ischaemia/reperfusion injury, and treatment with diuretics or vasodilators.15–19 Moreover, it has been described that treatment with cyclosporin, in comparison with previous treatment strategies without calcineurin blockers, results in less pronounced morphological and functional changes during acute rejection with loss of sensitivity of echocardiographic parameters.20 Furthermore, diastolic function parameters improve in the early postoperative period, reflecting recovery from the operative insult, which may conceal acute changes caused by the rejection process.4 Increased left ventricular mass in the absence of histological signs of acute rejection may also be the result of vascular (humoral) rejection, in which the cells of the microvasculature are targeted for injury early in the alloimmune response.7 However, because vascular rejection was not determined routinely in EMB, we were unable to evaluate the contribution of vascular rejection (alone or in combination with cellular rejection) to left ventricular wall thickening in our patients.
IL-2 mRNA expression in relation to rejection
IL-2 is a key regulator of cellular immunity by its autocrine stimulation of T cell maturation, differentiation, proliferation, and apoptosis.10,21 In agreement with our previous results, we found a strong relation between local IL-2 mRNA expression in EMB and histological signs of acute rejection.11 However, IL-2 mRNA is not always present during acute rejection and, inversely, acute rejection is not always diagnosed during the presence of IL-2 mRNA.14,22 Several factors may explain this discrepancy. Firstly, as a result of sampling errors the diagnosis of acute rejection may be false negative. Secondly, because of the redundancy of the cytokine network, lymphocyte proliferation can be stimulated by alternative T cell growth factors (such as IL-15) during inhibition of IL-2 mRNA expression by immunosuppressive therapy.23 Thirdly, IL-2 mRNA expression may precede histological and clinical signs of acute rejection or IL-2 mRNA can be expressed by a low number of infiltrated lymphocytes in the absence of myocyte damage (low grade rejection).24 Besides, although intragraft cytokine measurements do not automatically reflect peripheral events, we cannot entirely exclude the influence of bacterial or viral infection episodes on IL-2 mRNA expression within the graft. Finally, high concentrations of local IL-2 may trigger apoptosis of activated lymphocytes, thereby downregulating the immune response.21
Diastolic function in relation to IL-2 mRNA expression
New onset left ventricular dysfunction after heart transplantation may be caused by the presence and cytotoxic effects of infiltrating immune cells, but may also depend on the production of cytokines (such as IL-2) by these infiltrating cells.25 Cardiac complications are a common side effect of recombinant IL-2 immunotherapy in humans.13 Moreover, in the transplant setting, high IL-2 serum concentrations have been associated with impaired haemodynamics but were not correlated with impaired diastolic graft function as detected by echocardiography.26 Valantine and colleagues27 found no relation between IL-2 mRNA expression in EMB and acute rejection or allograft dysfunction. However, the measured IL-2 mRNA expression levels in their study were extremely low, probably because of the in situ hybridisation technique that they used.
In the present study, we found a significant relation between IL-2 positive acute rejection and increased left ventricular total wall thickness. This increase was not seen in patients with IL-2 positive AR− EMB or in patients with IL-2 negative acute rejections. We speculate that the IL-2 expression during acute rejection in our patient group may reflect the severity of the inflammation process during acute rejection. We did not observe a similar association with the measured functional parameters (E, A, deceleration time, and isovolumetric relaxation period), probably because of the large influence of loading conditions and blood pressure on these parameters. Our previous finding that IL-2 mRNA expression during the first acute rejection is associated with early development of graft vascular disease supports the hypothesis that IL-2 positive acute rejection episodes are more serious than IL-2 negative acute rejections.14 Because vascular leakage is a known side effect of IL-2 immunotherapy, it is likely that the wall thickening seen during IL-2 positive rejection episodes is the result of oedema.28 IL-2 may lead to oedema by stimulating the production of inducible nitric oxide synthase, leading to overproduction of nitric oxide, which has been reported to cause capillary damage followed by fluid leakage.29
In summary, our results show a significant increase of left ventricular total wall thickness before IL-2 positive acute rejection. Whether vascular rejection plays a part in this process remains to be determined. We conclude that rejection accompanied by intragraft IL-2 mRNA expression has a greater influence on heart morphology and therefore may be indicative of more severe rejection.
Abbreviations
AR+, in the presence of acute rejection
AR−, in the absence of acute rejection
cDNA, complementary DNA
E, peak early mitral flow velocity
EMB, endomyocardial biopsy
IL, interleukin
ISHLT, International Society for Heart and Lung Transplantation
mRNA, messenger RNA
PCR, polymerase chain reaction
REFERENCES
- 1.Ross HJ, Gullestad L, Hunt SA, et al. Early Doppler echocardiographic dysfunction is associated with an increased mortality after orthotopic cardiac transplantation. Circulation 1996;94:II289–93. [PubMed] [Google Scholar]
- 2.Valantine HA, Yeoh TK, Gibbons R, et al. Sensitivity and specificity of diastolic indexes for rejection surveillance: temporal correlation with endomyocardial biopsy. J Heart Lung Transplant 1991;10:757–65. [PubMed] [Google Scholar]
- 3.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. J Heart Transplant 1990;9:587–93. [PubMed] [Google Scholar]
- 4.Valantine HA. Rejection surveillance by Doppler echocardiography. J Heart Lung Transplant 1993;12:422–6. [PubMed] [Google Scholar]
- 5.Morocutti G, Di Chiara A, Proclemer A, et al. Signal-averaged electrocardiography and Doppler echocardiographic study in predicting acute rejection in heart transplantation. J Heart Lung Transplant 1995;14:1065–72. [PubMed] [Google Scholar]
- 6.Dodd DA, Brady LD, Carden KA, et al. Pattern of echocardiographic abnormalities with acute cardiac allograft rejection in adults: correlation with endomyocardial biopsy. J Heart Lung Transplant 1993;12:1009–18. [PubMed] [Google Scholar]
- 7.Gill EA, Borrego C, Bray BE, et al. Left ventricular mass increases during cardiac allograft vascular rejection. J Am Coll Cardiol 1995;25:922–6. [DOI] [PubMed] [Google Scholar]
- 8.Pulkki KJ. Cytokines and cardiomyocyte death. Ann Med 1997;29:339–43. [DOI] [PubMed] [Google Scholar]
- 9.Deng MC, Roeder N, Plenz G, et al. Proinflammatorische Zytokine und kardiale Pumpfunktion. Z Kardiol 1997;86:788–802. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen SL, Goldsmith MA, Greene WC. Interleukin-2 and the interleukin-2 receptor. In: Thomson AW, ed. The cytokine handbook. San Diego: Academic Press, 1998:73–103.
- 11.Baan CC, Weimar W. Intragraft cytokine gene expression: implications for clinical transplantation. Transplant Int 1998;11:169–80. [DOI] [PubMed] [Google Scholar]
- 12.Finkel MS, Oddis CV, Jacob TD, et al. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992;257:387–9. [DOI] [PubMed] [Google Scholar]
- 13.Fragasso G, Tresoldi M, Benti R, et al. Impaired left ventricular filling rate induced by treatment with recombinant interleukin 2 for advanced cancer. Br Heart J 1994;71:166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baan CC, Holweg CTJ, Niesters HGM, et al. The nature of acute rejection is associated with development of graft vascular disease after clinical heart transplantation. J Heart Lung Transplant 1998;17:363–73. [PubMed] [Google Scholar]
- 15.Mannaerts HF, Balk AH, Simoons ML, et al. Changes in left ventricular function and wall thickness in heart transplant recipients and their relation to acute rejection: an assessment by digitised M mode echocardiography. Br Heart J 1992;68:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannaerts HF, Simoons ML, Balk AH, et al. Pulsed-wave transmitral Doppler do not diagnose moderate acute rejection after heart transplantation. J Heart Lung Transplant 1993;12:411–21. [PubMed] [Google Scholar]
- 17.Fauchier L, Sirinelli A, Aupart M, et al. Performances of Doppler echocardiography for diagnosis of acute, mild, or moderate cardiac allograft rejection. Transplant Proc 1997;29:2442–5. [DOI] [PubMed] [Google Scholar]
- 18.Spes CH, Schnaack SD, Schütz A, et al. Serial Doppler echocardiographic assessment of left and right ventricular filling for non-invasive diagnosis of mild acute cardiac allograft rejection. Eur Heart J 1992;13:889–94. [DOI] [PubMed] [Google Scholar]
- 19.Janssen PM, Zeitz O, Keweloh B, et al. Influence of cyclosporine A on contractile function, calcium handling, and energetics in isolated human and rabbit myocardium. Cardiovasc Res 2000;47:99–107. [DOI] [PubMed] [Google Scholar]
- 20.Kemkes BM, Schütz A, Engelhardt M, et al. Noninvasive methods of rejection diagnosis after heart transplantation. J Heart Lung Transplant 1992;11:S221–31. [PubMed] [Google Scholar]
- 21.Lenardo M, Chan KM, Hornung F, et al. Mature T lymphocyte apoptosis: immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol 1999;17:221–53. [DOI] [PubMed] [Google Scholar]
- 22.Normann SJ, Peck AB, Staples ED, et al. Experimental and clinical allogeneic heart transplant rejection: correlations between histology and immune reactivity detected by cytokine messenger RNA. J Heart Lung Transplant 1996;15:778–89. [PubMed] [Google Scholar]
- 23.Baan CC, Knoop CJ, van Gelder T, et al. Anti-CD25 therapy reveals the redundancy of the intragraft cytokine network after clinical heart transplantation. Transplantation 1999;67:870–6. [DOI] [PubMed] [Google Scholar]
- 24.Dallman MJ, Roake J, Hughes D, et al. Sequential analysis of IL-2 gene transcription in renal transplants. Transplantation 1992;53:683–5. [DOI] [PubMed] [Google Scholar]
- 25.McNamara D, Di Salvo T, Mathier M, et al. Left ventricular dysfunction after heart transplantation: incidence and role of enhanced immunosuppression. J Heart Lung Transplant 1996;15:506–15. [PubMed] [Google Scholar]
- 26.Deng MC, Erren M, Kammerling L, et al. The relation of interleukin-6, tumor necrosis factor-α, IL-2, and IL-2 receptor levels to cellular rejection, allograft dysfunction, and clinical events early after cardiac transplantation. Transplantation 1995;60:1118–24. [DOI] [PubMed] [Google Scholar]
- 27.Valantine H, Johnson F, Dong C, et al. Cytokines as potential mediators of acute allograft diastolic dysfunction in cyclosporine-treated patients: a pilot study using in situ hybridization. Transplant Proc 1994;26:2852–3. [PubMed] [Google Scholar]
- 28.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 1997;37:117–32. [DOI] [PubMed] [Google Scholar]
- 29.Orucevic A, Lala PK. Role of nitric oxide in IL-2 therapy-induced capillary leak syndrome. Cancer Metastasis Rev 1998;17:127–42. [DOI] [PubMed] [Google Scholar]