Abstract
A case is presented of the Churg-Strauss syndrome with hypereosinophilia and severe cardiac involvement, namely biventricular endomyocardial fibrosis and gross encroachment of the right ventricular cavity. The clinical picture was similar to Loeffler's syndrome and the idiopathic hypereosinophilic syndrome. Combined aggressive surgical and medical management led to full recovery and survival at 10 years. The good long term outcome is attributed to strict control of peripheral eosinophil count by oral corticosteroids. This case illustrates the damaging effects of hypereosinophilia on the heart.
Keywords: Churg-Strauss syndrome, cardiac involvement, hypereosinophilia
The Churg-Strauss syndrome is an inflammatory disease characterised by asthma and granulomatous angiitis together with tissue and blood eosinophilia. A prodromal phase has been described, with asthma and rhinitis, succeeded by an infiltrative eosinophilic phase with similarities to Loeffler's syndrome, and finally a severe vasculitic phase.1 Cardiac complications have a major role in mortality and morbidity; endomyocardial fibrosis is described though rare. We present a patient with complete resolution of severe cardiac manifestations and prolonged survival after combined surgical and medical management.
CASE REPORT
A woman with a history of atopy and nasal polyposis developed asthma in 1985, aged 48 years. Symptoms were satisfactorily controlled for three years with inhaled salbutamol and beclomethasone, until a deterioration with pulmonary infiltrates and blood eosinophilia (6 × 109/l). She required oral prednisolone at a variable dose over the next few years to control symptoms and the eosinophil count ranged between 0.6–4.8 × 109/l. In March 1991 the patient became systemically unwell with fever, weight loss, and malaise. The eosinophil count rose to a maximum of 12 × 109/l. She developed right heart failure, a left retinal infarct attributed to vasculitis, and bilateral foot drop with electrophysiological evidence of mononeuritis multiplex.
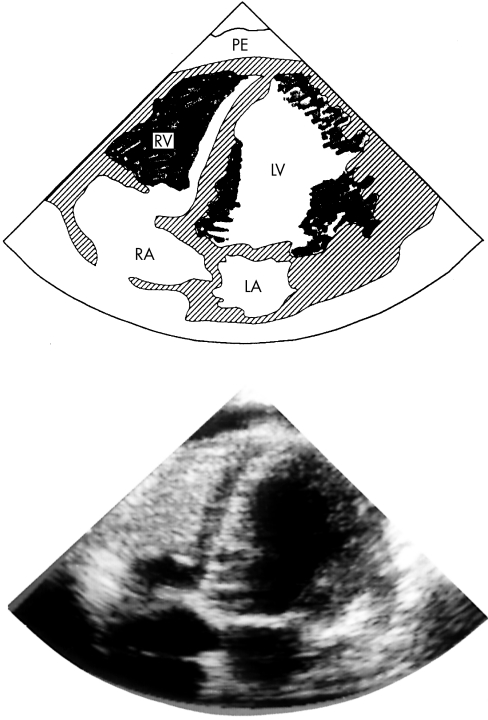
Renal function and serum IgE were normal. The echocardiogram (fig 1) showed gross encroachment into the right ventricular cavity by an echo dense mass and further, though less extensive, involvement of the left ventricle. Contractility was severely impaired and a pericardial effusion was present. Myocardial biopsy showed histological changes in keeping with endomyocardial fibrosis, with diffuse infiltration between myocytes of young connective tissue and some haemosiderin deposition.
Figure 1.
Echocardiogram revealing almost complete replacement of the right ventricular cavity, leaving a very small channel from the right atrium into the pulmonary artery. There is involvement of the left ventricle. The diagram shows the infiltration shaded in black.
Because of poor cardiac output the patient underwent open heart surgery. The right ventricle was opened and the cavity was found to be almost completely replaced by layers of well organised clot on the surface and yellow gelatinous fibrin about 3 mm thick over the myocardium, extending with a fairly clear line of demarcation from about 1 cm from the atrioventricular ring to about 1 cm of the pulmonary artery. The tricuspid chordae passed through the clot and the abnormal endocardial tissue adhered firmly to all the trabeculae. There was a plain between the muscle and the abnormal tissue but the tissue extended deeply into every crevice over the trabeculae. It was removed in numerous chippings very much like decorticating an empyema. By this means the bulk was substantially reduced. The tricuspid valve was replaced by a Carpentier Edwards xenograft. The left ventricle was not opened.
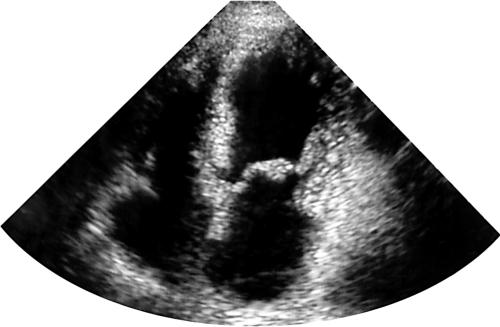
Postoperatively, she received corticosteroids, warfarin, azathioprine, aspirin, and dipyridamole. Progress was slow but the heart failure, visual impairment, and neuropathy resolved. It was possible to simplify the regimen to warfarin and prednisolone, the dose averaging about 5 mg daily and being adjusted carefully to maintain a normal blood eosinophil count. She has continued to require inhaled asthma medication. She remains in good health at 10 years after surgery and the echocardiogram is now normal (fig 2).
Figure 2.
Ten years after surgery, echocardiography shows no evidence of infiltration.
DISCUSSION
This patient fulfils the criteria for the Churg-Strauss syndrome1, 2 despite a lack of histological evidence of angiitis. She also had hypereosinophilia by the definition of blood eosinophilia of > 1.5 × 109/l. The case is of note because of the severe encroachment of abnormal tissue into the cavity of the right ventricle, the description of the operative findings and procedure, and the excellent clinical outcome at 10 years. Of particular interest is the resolution of the left ventricular masses with immunosuppressive treatment alone. Although surgery was critical to the patient's early survival, we attribute her good long term progress to meticulous control of the inflammatory process with oral steroids, using the blood eosinophil count as the marker of disease activity.
Cardiac involvement in Churg-Strauss syndrome is well described. Manifestations include coronary vasculitis, acute and constrictive pericarditis, myocardial fibrosis, and cardiac failure.1 Endomyocardial fibrosis similar to Loeffler's syndrome as seen in this case has been recorded3 but is rare. This pattern of cardiac involvement has been more usually described in the idiopathic hypereosinophilic syndrome4 and the mechanisms by which eosinophilia may damage the heart are discussed by Spry.5 From their granules, eosinophils release proteins, including ribonucleases and eosinophil major basic protein, that attack parasites. These substances damage cardiac myocytes but not skeletal muscle.
Some authors consider that hypereosinophilic syndrome is a separate entity from Churg-Strauss syndrome, being defined as hypereosinophilia for more than six months in the absence of other recognised causes of hypereosinophilia. It seems more probable that there are overlap syndromes and that the cardiac damage is caused by the toxic effects of hypereosinophilia rather than by the underlying disease process. This leads to the conclusion that control of the blood eosinophil count in these syndromes is vital for cardiac health.
REFERENCES
- 1.Lanham JC, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine 1984;63:65–81. [DOI] [PubMed] [Google Scholar]
- 2.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of the Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094–101. [DOI] [PubMed] [Google Scholar]
- 3.Lanham JC, Cooke S, Davies J, et al. Endomyocardial complications of the Churg-Strauss syndrome. Postgrad Med J 1985;61:341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies J, Sapsford R, Brooksby I, et al. Successful surgical treatment of two patients with eosinophilic endomyocardial disease. Br Heart J 1981;46:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spry CJF. The hypereosinophilic syndrome and the heart. In: Weatherall DJ, Ledingham JGG, Warrell DA, eds. The Oxford textbook of medicine, 3rd ed. Oxford: Oxford University Press, 1996:2396–8.