Abstract
Objective: To evaluate the clinical and electrophysiological determinants of arrhythmia recurrence in patients undergoing internal atrial cardioversion for chronic atrial fibrillation (AF).
Setting: Tertiary cardiac referral centre.
Methods: 101 consecutive patients with failed external cardioversion or AF ≥ 1 year underwent internal atrial cardioversion; once stable sinus rhythm (SR) was obtained, electrophysiological study was performed in 73 patients (72%) who gave informed consent. Patients were then followed on antiarrhythmic treatment.
Results: 101 consecutive patients underwent internal atrial cardioversion in the period 1996–1999 with 100% conversion to SR; prophylactic antiarrhythmic treatment was flecainide (52%), amiodarone (37%), and sotalol (11%). Average follow up at first AF recurrence was 18.4 (14.4) months (range 0.1–49.8 months); persistence of SR was observed in 72/101 (72%) patients. By logistic regression, AF duration (odds ratio (OR) 1.07, 95% confidence interval (CI) 1.01 to 1.13) and a lower sinus rate at discharge on antiarrhythmic drugs (OR 0.92, 95% CI 0.85 to 0.99) were independent predictors of AF recurrence, whereas age, New York Heart Association functional class, left atrial dimensions, and left ventricular ejection fraction were not predictive of arrhythmia recurrence. When electrophysiological parameters were added to the statistical model in 73 patients, a shorter atrial effective refractoriness (OR 1.04, 95% CI 1 to 1.08) and an abnormal relation of atrial effective refractoriness to cycle length (OR 31, 95% CI 3.7 to 266) were also independent predictors of AF recurrence at follow up, beyond AF duration and heart rate at discharge.
Conclusions: Patients with failed external cardioversion or long lasting AF may benefit from internal atrial cardioversion and antiarrhythmic treatment to keep SR at long term; electrophysiological study may identify patients at the highest risk of arrhythmia recurrence. Although preservation of SR seems unlikely for AF duration > 3 years, a consistent minority of this subgroup (38%) may benefit from this approach.
Keywords: atrial fibrillation, internal atrial cardioversion, electrophysiology, follow up
Internal atrial cardioversion is a useful technique to restore sinus rhythm (SR) in patients for whom external electrical cardioversion is unsuccessful or with chronic atrial fibrillation (AF) of long duration, with an efficacy ranging from 70–90%.1,2 Once cardioversion is successful, preservation of SR for the long term is the main treatment goal; unfortunately, despite the use of antiarrhythmic drugs and serial cardioversion, the rate of AF recurrence ranges from 40–50% in the first year.3–5 Long AF duration, older age, and relevant heart disease are recognised as being predictive of AF recurrence at follow up5,6; indeed, experimental data suggest that electrophysiological changes take place with long lasting AF, which in turn promote AF relapse and chronic AF in the long term.7
Our study goals were to investigate the clinical and electrophysiological determinants of AF recurrence in a patient population at high risk of AF relapse (patients with chronic AF who were candidates for internal atrial cardioversion after failed external cardioversion or with AF lasting ≥ 1 year) and to test the hypothesis that maximised antiarrhythmic treatment may achieve favourable results after cardioversion.
METHODS
From January 1996 to December 1999, consecutive patients with chronic AF referred to our centre after failed external electrical cardioversion or with AF lasting longer than one year were offered internal atrial cardioversion. Cardioversion was not proposed to patients with an expected low likelihood to maintain SR: history of recurrent drug refractory AF or AF caused by advanced and unstable heart disease or severe concurrent medical illnesses. In this latter subgroup SR is short lived, whereas AF does not seem to be associated with poorer prognosis8; thus, repeated cardioversion attempts have little clinical value.
Patients were not taking antiarrhythmic drugs other than those for ventricular rate control (calcium channel blockers, β blockers, and digoxin), which were withdrawn 48 hours before cardioversion (72 hours for digoxin). The methods for internal atrial cardioversion in our centre have been described in detail elsewhere.9 Briefly, biphasic shocks (3 ms/3 ms duration), R wave synchronised by a right ventricular bipolar lead, were delivered through two identical coil catheters with a 4.63 cm2 electrode surface area (Rhythm Technologies, Jacksonville, Florida, USA) placed in the right atrium and the coronary sinus. The shock strength was increased in 60 V increments from 180 V up to a maximum of 600 V. General anaesthesia was never required for patients' discomfort, as reported in several studies1,9,10; mild sedation was administered at the patient's request to 27 of 101 (27%) patients. Patients undergoing internal atrial cardioversion were asked to participate in an investigational study, which required an additional electrophysiological evaluation (EP) at resumption of SR and written informed consent. The EP was performed at the high right atrium after 10 minutes of stable SR and comprised measurement of corrected sinus node recovery time, determination of atrial effective refractory period (ERP) at multiple cycle lengths (CLs), and determination of the relation of ERP to CL. ERP was calculated following an incremental protocol: the coupling interval of the extrastimulus was increased from a minimum of 100 ms in 10 ms steps until atrial capture was elicited, then 1 ms incremental steps were applied starting from a 20 ms shorter coupling interval until atrial capture was consecutively confirmed thrice. If AF was induced during EP, cardioversion was achieved immediately and a new EP was started again after 10 minutes. The relation of ERP to CL in healthy subjects had been previously evaluated in a group of 15 patients undergoing EP, without a clinical history of AF or AF inducibility at EP. ERP was measured at the intrinsic (“basal”) cycle length (BCL) and at four decreasing CLs: 600 ms, 500 ms, 400 ms, and 330 ms. The difference between the maximum and minimum ERPs through these five CLs was defined as ΔERP. The relation of ERP to CL in each patient with AF was prospectively defined using data obtained from the control subjects as follows: normal, when ΔERP through the five CLs was greater than 20 ms and ERP shortened at decreasing CLs from BCL to 330 ms; abnormal, when ΔERP was ≤ 20 ms or ERP increased at decreasing CLs from BCL to 330 ms.
Internal cardioversion was deemed successful when the patient was able to leave the electrophysiology laboratory in stable SR. Spontaneous AF recurrences and AF inductions during the EP protocol were terminated by further cardioversion and were not regarded as follow up events.
To minimise early AF recurrences, intravenous pharmacotherapy was started as soon as the procedure ended.
Drug selection was based on the clinical characteristics of the patients: severity of heart failure symptoms, degree of left ventricular dysfunction, and coexistence of coronary artery disease.
Flecainide was prescribed only to those with a left ventricular ejection fraction ≥ 45%, in New York Heart Association (NYHA) functional class ≤ II, and without coronary artery disease. Sotalol was prescribed to patients in NYHA class ≤ II, preferably with coexistent coronary artery disease. Amiodarone was given to patients with relevant heart failure (NYHA class > II) caused by systolic dysfunction.
Drug administration was started as an intravenous loading at the end of the EP (73 patients) or at SR resumption (28 patients): flecainide up to 2 mg/kg, sotalol up to 1.5 mg/kg, and amiodarone up to 5 mg/kg. Dose titration relied on the patients' characteristics (body weight, lean weight, renal function) and serial ECG measurements. Drug effects were monitored by serial ECG measurements following customary guidelines of our centre and data from the literature.11
Flecainide was titrated so as not to exceed a 30% increase over the baseline QRS duration and prolongation of the QTc interval > 520 ms to avoid proarrhythmic ventricular events12 up to a maximum of 400 mg according to patient tolerance.
Sotalol was increased to ensure consistent dosage following QTc interval prolongation (maximum 520 ms) and QTc dispersion, while looking for abnormal repolarisation changes to avoid proarrhythmic ventricular events, up to a maximum of 480 mg according to patient tolerance.13–15
Amiodarone was administered as an intravenous loading of 1.8 g/24 hours, then 800 mg daily for one week, 600 mg daily for one month, and 400 mg daily for four months before dosage tapering. The pharmacological effect was evaluated by repolarisation changes16; abnormal repolarisation or a QTc interval prolongation > 520 ms indicated dosage tapering at any time.15
All the patients were closely monitored for the onset of worsening heart failure symptoms, electrolyte unbalance, and pathological bradycardia during the hospital stay and at follow up visits.
Statistical analysis
Electrophysiological characteristics of control and AF patients were compared by unpaired t test. Logistical regression was performed to assess clinical and electrophysiological correlates of AF recurrence. We tested the following variables for all patients: age, sex, body surface area, NYHA functional class, left ventricular ejection fraction, left atrial dimension, and resting heart rate while on antiarrhythmic drugs at discharge. Logistical regression was also performed on data from the subgroup who agreed to undergo EP. In addition to the aforementioned parameters, ERP at BCL and relation of ERP to CL were included in the statistical model. Kaplan-Meier curves were constructed and compared by log rank to provide assessment of the prognostic value of predictors of AF recurrence. The statistical package used was SPSS version 9.0 (SPSS Inc, Chicago, Illinois, USA). All parameters are presented as mean (SD).
RESULTS
Fifteen patients without a clinical history of AF and no AF inducibility at EP served as controls for corrected sinus node recovery time, ERP, ΔERP, and ERP relation to CL. These 15 patients were aged 61 (9) years on average ( range 32–73) and had a left ventricular ejection fraction of 59 (15)% (range 27–75%). Four patients had atrioventricular node re-entrant tachycardia, two patients had supraventricular tachycardia caused by a concealed accessory pathway, three patients had unexplained syncope, and six patients had monomorphic ventricular tachycardia.
In all of these patients ERP shortened at decreasing CLs from BCL to 330 ms (table 1); ΔERP averaged 34 ms ( range 24–44).
Table 1.
Electrophysiological characteristics of control subjects and patients with atrial fibrillation (AF)
| BCL (ms) | Control group | AF group | |
| cSNRT (ms) | 397 (54) | 386 (39) | |
| Range | 265–490 | 272–440 | |
| BCL (ms) | 996 (137) | 802 (104)** | |
| Range | 820–1150 | 560–993 | |
| ERP (ms) | 218 (9) | 184 (24)** | |
| Range | 209–236 | 126–230 | |
| ERP (ms) | 600 | 207 (5) | 183 (23)** |
| Range | 200–218 | 130–240 | |
| ERP (ms) | 500 | 198 (6) | 179 (18)** |
| Range | 190–210 | 140–225 | |
| ERP (ms) | 400 | 183 (6) | 174 (16)* |
| Range | 178–190 | 130–215 | |
| ERP (ms) | 330 | 178 (5) | 168 (17)* |
| Range | 172–190 | 140–212 | |
| ΔERP (ms) | 34 (6) | 19 (13)** | |
| Range | 24–44 | 0–52 |
Data are mean (SD).
*p<0.05; *p<0.001.
BCL, basal cycle length; cSNRT, corrected sinus node recovery time;
ERP, atrial refractory period; ΔERP, difference between maximum and minimum atrial refractory periods.
One hundred and one patients underwent internal atrial cardioversion, 55 (55%) because of failed external cardioversion and 46 (45%) because of AF lasting longer than one year; 73 of 101 (72%) agreed to participate in the EP protocol. Underlying disease was hypertensive heart disease (40 patients), dilated cardiomyopathy (18 patients), valvar heart disease (16 patients), coronary artery disease (11 patients), previous thyrotoxicosis (7 patients), diabetes (3 patients), and lone AF (6 patients). Table 2 lists the main clinical parameters.
Table 2.
Study population and predictors of AF recurrence based on logistic regression analysis
| Variable | Overall (n=101) | SR (n=72) | AF (n=29) | OR | 95% CI |
| Age (years) | 61 (11) | 63 (10) | 59 (13) | 0.98 | 0.94 to 1.03 |
| Range | 30–81 | 30–81 | 37–76 | ||
| Male/female | 62/39 | 44/28 | 18/11 | 0.8 | 0.52 to 1.13 |
| BSA (m2) | 1.88 (.19) | 1.86 (.2) | 1.94 (.17) | 0.2 | 0.02 to 3.4 |
| Range | 1.37–2.3 | 1.37–2.3 | 1.64–2.26 | ||
| NYHA II | 77 (78%) | 55 (76%) | 22 (75%) | 1.06 | 0.25 to 4.49 |
| NYHA >II: III | 21 (20%) | 15 (21%) | 6 (21%) | ||
| IV | 3 (2%) | 2 (3%) | 1 (4%) | ||
| EF (%) | 56 (14) | 56 (13) | 56 (14) | 1.00 | 0.96 to 1.05 |
| Range | 18–81 | 18–76 | 30–81 | ||
| LA (mm) | 48 (6.6) | 47 (6) | 50 (7.3) | 1.03 | 0.95 to 1.12 |
| Range | 33–66 | 33–66 | 38–72 | ||
| AF duration (months) | 15 (20) | 11 (13) | 26 (29) | 1.04 | 1.01 to 1.07* |
| Range | 3–120 | 3–61 | 3–120 | ||
| HR (beats/min) | 61 (10) | 63 (10) | 57 (9) | 0.92 | 0.88 to 0.98** |
| Range | 35–87 | 41–87 | 35–84 | ||
| Sotalol | 11 | 6 | 5 | 2831 | 0.00 to 2.5722 |
| Amiodarone | 37 | 28 | 9 | 1366 | 0.00 to 1.222 |
| Flecainide | 53 | 38 | 15 | 1051 | 0.00 to 9.1721 |
Data are mean (SD).
*p=0.01; **p=0.007.
BSA, body surface area; CI, confidence interval; EF, left ventricular ejection fraction; HR, heart rate at discharge on antiarrhythmic drugs; LA, left atrial diameter; NYHA, New York Heart Association functional class; OR, odds ratio; SR, sinus rhythm.
SR was restored in all the patients. There was one spontaneous recurrence of AF and sustained AF was induced during EP in 13 patients (in four of these, AF was induced twice). All these AF episodes were terminated by repeated cardioversion. No patient developed intractable recurring AF, as a result of patient selection. In fact, all but three patients were in stable NYHA class II or III and none were expected according to their clinical history to have drug refractory AF.
The strength of effective shocks ranged from 180–600 V (2–24.2 J) with a mean of 353 (84) V (8.5 (4.7) J) and a median of 370 V (7.5 J). Shocks of 358 (89) V (9 (5) J) were used for patients who maintained SR and 341 (72) V (7.7 (3.7) J) for those with AF recurrence (NS).
Fifty three patients (52%) were discharged on flecainide, 37 received amiodarone (37%), and 11 received sotalol (11%). The drug dose was carefully adjusted for each patient based on serial ECG measurement of PR, QRS, and QTc intervals and individual tolerance: 272 (46) mg (range 150–350 mg, median 300 mg) for flecainide; 320 (50) mg (range 240–400 mg, median 320 mg) for sotalol; and 600 mg daily for one month and 400 mg daily for four months before dosage tapering for amiodarone.
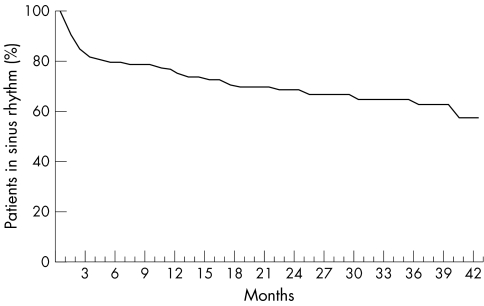
Patients were followed at months 1, 3, 6, 12, 18, and 24 and then yearly after cardioversion. Average follow up time was 18 (14) months (range 0.1–49.8). Follow up was 22 (13) months (range 3.8–49.8) for patients who maintained SR. Time to first AF recurrence was 7.8 (10) months (range 0.1–38) for those with AF relapse (p < 0.001). Fig 1 shows the probability of freedom from AF recurrence.
Figure 1.
Probability of freedom from atrial fibrillation recurrence during follow up.
Overall, the rate of AF recurrence was 29% (29 of 101 patients); broken down by antiarrhythmic treatment at discharge, it was 28% for flecainide (15 of 53), 24% for amiodarone (9 of 37), and 45% for sotalol (5 of 11). During follow up, 6 of 29 (21%) recurrences occurred within one week and 13 of 29 (45%) in the first month (fig 1).
Among these 101 patients, only duration of AF before cardioversion, heart rate at discharge while the patient was taking antiarrhythmic drugs, and use of sotalol were significantly associated with AF recurrence by logistic regression. Age, sex, BSA, NYHA class, left ventricular ejection fraction, and left atrial diameter, were not predictive of patient outcome (table 2).
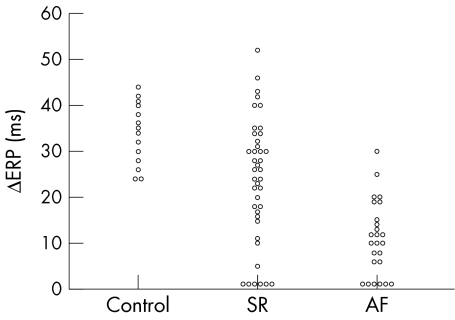
Of these 101 patients, 73 agreed to participate in the EP protocol. The main clinical variables did not differ from the 101 population as a whole; table 3 lists these variables together with EP derived parameters. Fig 2 shows the type of ERP relation with CL (ΔERP) for patients who maintained SR at follow up and for patients with AF recurrence compared with control patients.
Table 3.
Predictors of AF recurrence in patients undergoing electrophysiological study
| Variable | Overall (n=73) | SR (n=47) | AF (n=26) | OR | 95% CI |
| Age (years) | 62 (10) | 64 (10) | 60(12) | 0.96 | 0.9 to 1.03 |
| Range | 30–81 | 30–81 | 35–76 | 0.83 | 0.55 to 1.14 |
| Male/female | 43/30 | 28/19 | 15/11 | ||
| BSA (m2) | 1.88 (.19) | 1.88 (.2) | 1.88 (.18) | 0.8 | 0.01 to 4.3 |
| Range | 1.37–2.26 | 1.37–2.22 | 1.56–2.26 | ||
| NYHA II | 53 (73%) | 33 (70%) | 20 (76%) | 1.95 | 0.26 to 14.8 |
| NYHA III | 20 (27%) | 14 (30%) | 6 (24%) | ||
| EF (%) | 56 (14) | 56 (14) | 55 (13) | 0.97 | 0.91 to 1.03 |
| Range | 18–86 | 18–81 | 30–86 | ||
| LA (mm) | 48 (7) | 47 (6) | 49 (8) | 0.96 | 0.85 to 1.09 |
| Range | 31–72 | 33–66 | 31–72 | ||
| AF duration (months) | 15 (21) | 12 (14) | 25 (28) | 1.07 | 1.01 to 1.13** |
| Range | 3–120 | 3–61 | 3–120 | ||
| HR (beats/min) | 62 (10) | 65 (9) | 56 (11) | 0.92 | 0.85 to 0.99* |
| Range | 35–87 | 44–87 | 35–84 | ||
| cSNRT (ms) | 437 (78) | 428 (73) | 441 (80) | 0.94 | 0.82 to 1.06 |
| Range | 290–560 | 310–520 | 290–550 | ||
| ERP at BCL (ms) | 184 (21) | 188 (18) | 180 (22) | 1.04 | 1 to 1.08* |
| Range | 126–230 | 142–230 | 126–230 | ||
| Abnormal ERP/CL (patients) | 42/73 | 18/47 | 24/26 | 31.5 | 3.7 to 266*** |
Data are mean (SD).
*p=0.02; **p=0.007; ***p=0.001.
Abnormal ERP/CL, abnormal relation of atrial ERP to cycle length; LA, left atrium diameter; SR, sinus rhythm.
Figure 2.
Relation of atrial effective refractory period (ERP) to cycle length in control patients (Control), in patients who maintained sinus rhythm (SR), and in patients with atrial fibrillation recurrence (AF). An abnormal relation is observed when the difference in maximum and minimum ERP (ΔERP) ≤ 20 ms.
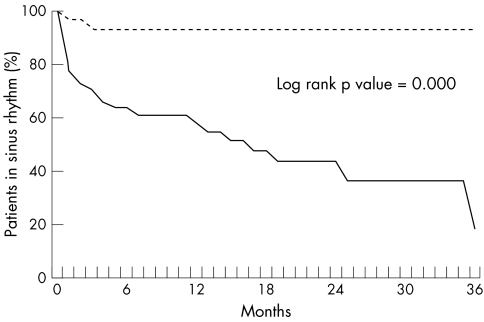
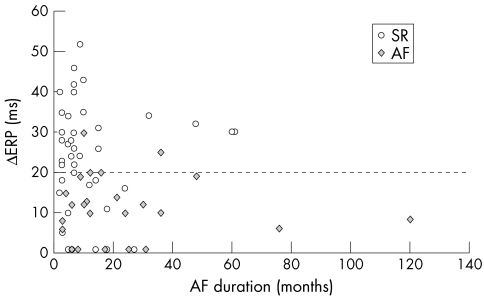
AF recurred in 26 of 73 patients (36%); 47 of 73 (64%) were in SR at the last follow up visit. Among these 73 patients, longer AF duration, lower heart rate, a shorter ERP at BCL, and an abnormal relation of ERP to CL were significantly associated with AF recurrence by logistic regression analysis (table 3). The other variables were not predictive of AF recurrence. Fig 3 shows the freedom from AF recurrence as a function of the ERP relation to CL (Kaplan-Meier curve). An abnormal ERP relation to CL (ΔERP) is plotted against AF duration in fig 4 for patients who maintained SR and for those who relapsed into AF.
Figure 3.
Likelihood of atrial fibrillation recurrence as a function of the relation of ERP to cycle length (a normal relation is shown by the broken line and an abnormal relation by the continuous line).
Figure 4.
Relation of ERP to cycle length plotted against atrial fibrillation duration for patients who maintained sinus rhythm (SR) and for those who had atrial fibrillation recurrence (AF).
During follow up five patients died: at 1 and 10 months (heart failure), 12 months (cancer), 19 months (stroke), and 24 months (respiratory failure). Only the first and the third of these five were in AF at the time of death. Of 96 living patients, 69 were in SR (72%) and 27 (28%) were in AF at their last follow up visit.
Among 46 patients with AF duration ≥ 1 year (31.7 (24.6) months, range 12–120 months), 29 (63%) were in SR. Among 13 patients with AF ≥ 3 years (61.6 (24.6) months, range 36–120 months) only five (38%) were in SR at their last follow up visit.
During follow up, no sudden deaths occurred. New ventricular arrhythmias and AF with 1:1 synchronisation at fast ventricular rates were never observed.
DISCUSSION
AF recurrence is the most threatening event after cardioversion to SR, occurring in up to 80% of patients at one year in the absence of antiarrhythmic treatment,5 which may lower this recurrence rate to 40–50% at one year.3,5 Recurrence of AF exposes patients to additional discomfort and cardiovascular events, and leads to hospitalisations and other interventions that impose a relevant economic burden on health care systems. In this view, reliable means to identify patients most likely to relapse would be greatly beneficial to clinical practice. According to Van Gelder and colleagues,4,5 clinical variables such as AF duration ≥ 3 months, age over 56 years, and NYHA class ≥ III or relevant heart disease are associated with AF relapse in the long term in a study using drug prophylaxis and serial cardioversion in case of AF recurrence. Nonetheless, once patients at low risk of recurrence (age < 56 years, no heart disease, a single AF episode of short duration) have been identified, individual differences in the risk of AF recurrence are wide among subjects with one or more of these predisposing characteristics.
On the basis of this background, we considered patients at relatively high risk of AF recurrence with relevant heart disease (84% of patients) and we tested the hypothesis that EP may aid in identifying patients at risk of AF relapse. Our observations confirm that AF duration is an independent predictor of recurrence, as previously reported.5,6 On the other hand, ejection fraction and NYHA class (relatively well preserved in our population), left atrial diameter, and other clinical variables were not associated with outcome. When EP derived parameters were added to the statistical model (logistic regression) of 73 patients, the highest relative risk for recurrence was observed with an abnormal ERP relation to CL. AF duration, heart rate, and atrial ERP were also predictive of recurrence but to a lesser degree (table 3, fig 3).
An abnormal ERP relation to CL seems to be the consequence of long lasting AF in the experimental model in goats7; indeed, it has been reported to be a strong predisposing factor to AF in human myocardium17 as well as in the clinical setting.18,19 Manios and colleagues19 recently reported that the only electrophysiological indicator of AF recurrence following internal cardioversion was an abnormal rate adaptation of atrial action potential duration in 28 patients with chronic AF not treated with antiarrhythmic drugs. In our study, rate maladaptation of ERP held the highest odds ratio for AF relapse (table 3), which was independent of AF duration, as shown in fig 4. Moreover, in our population no cut off value of AF duration was found above which only an abnormal relation of ERP to CL was observed. It is then conceivable that within a time frame of AF duration of 60 months, maladaptation of refractoriness to CL is more patient dependent than merely AF duration dependent. That is, individual characteristics of the patient or of the arrhythmogenic substrate are more prominent than arrhythmia duration.
Short atrial refractoriness has also been reported to be a vulnerable parameter predisposing patients to AF in clinical studies.20,21 Progressive shortening of ERP caused by sustained AF episodes has indeed been shown in goats7 and in humans,22 suggesting that shortening of refractoriness may be responsible for perpetuating AF. This finding becomes clinically relevant for AF episodes of considerable duration (hours to days in goats), whereas it appeared to be reversible within 10 minutes when short lived AF was induced during EP in humans.22 Recent observations have elucidated the time course of recovery of atrial refractoriness and action potential duration following cardioversion of chronic AF: Manios and colleagues19 observed that both parameters recover to values comparable with those of healthy subjects 24 hours after cardioversion. Short atrial refractoriness may reasonably have a pivotal role in AF recurrences within the first few days after cardioversion, whereas after this period its relevance remains to be elucidated.
The observation that inhomogeneity of atrial refractoriness promotes AF has been reported in several studies,23,24 although with important technical limitations, mainly in patients with paroxysmal AF or AF induced during EP (a very different condition from chronic AF). On the contrary, De Sisti and colleagues25 found no difference of atrial refractoriness between control subjects and patients with sick sinus node disease with or without AF. Indeed, inhomogeneity of atrial refractoriness may at least partly result from peculiar autonomic activity,26 which was not evaluated in these studies. Moreover, patients with chronic AF have a high likelihood of repeated AF inductions during electrophysiological measurements,27 which is a major obstacle to accurate multisite evaluations. For this reason we relied on single site determination of atrial refractoriness, as reported in most experimental and clinical studies.7,18–22,27 The role of atrial refractoriness inhomogeneity seems to require further investigation.
A short refractory period and its poor adaptation to heart rate are viewed as the electrophysiological milieu (termed “electrophysiological remodelling”) for AF recurrence: maladaptation of refractoriness to CL and short ERP cause a wide window of vulnerability to AF triggers throughout the cardiac cycle, such as ectopic beats, and if significant bradycardia or chronotropic incompetence coexists, that window becomes progressively wider as CL increases. In our study, the observation that a lower heart rate in patients taking drugs was predictive of AF recurrence is indeed consistent with this concept. These findings have important clinical implications when therapeutic interventions are considered to avoid bradycardia disproportionate to the increase in ERP; furthermore, this aspect may be useful in designing the pharmacodynamic profile of new antiarrhythmic drugs. In this perspective, knowledge of the ionic mechanisms of electrophysiological remodelling may also guide pharmacological prevention of AF recurrences: experimental studies have shown that shortening of atrial action potential duration and effective refractoriness during AF are limited by the T type calcium channel blocker mibefradil, whereas no effect is exerted by L type calcium channel blockers (verapamil, diltiazem).28,29
The complex interplay between abnormal ERP relation to CL and heart rate may also stimulate speculation about the unexpected performance of sotalol compared with flecainide and amiodarone; in fact, five of five patients who relapsed on sotalol had maladaptation of ERP to CL. Given the prognostic weight of rate maladaptation of ERP, the major bradycardia caused by sotalol may have been detrimental in this subgroup from an electrophysiological point of view.
Interestingly, a higher recurrence rate (80%) among patients given sotalol after internal atrial cardioversion of long lasting AF (≥ 1 year) than among those given amiodarone (40%) has been reported by Tse and colleagues.6 However, no EP data or heart rate at discharge are available from that study, thus leaving the issue about the role of heart rate and of rate dependent effects of class III antiarrhythmic drugs open to investigation.
In the same study, Tse and colleagues6 observed that AF duration was the only powerful predictor of arrhythmia recurrence: when the population was dichotomised into AF duration ≥ 3 years and < 3 years the recurrence rates were 89% and 55%, respectively, with most of the relapses occurring within the first month. In our study, 38% of patients with AF duration ≥ 3 years were in SR (nine on flecainide, two on sotalol, and two on amiodarone), raising the point that a sizeable minority of patients may still benefit from attempted cardioversion.10 To prevent early AF relapse, vigorous antiarrhythmic treatment has been recommended for patients at high risk,5,6,9 whereas “hybrid” treatment (a combination of pharmacological and non-pharmacological therapy) is the emerging concept for patients who relapse in spite of antiarrhythmic drugs.30,31 From this perspective, our study offers reliable parameters to identify high risk patients soon after cardioversion to guide antiarrhythmic treatment following internal atrial cardioversion or to find clues to the optimal additional treatment.
In conclusion, EP is a reliable risk stratification tool and EP guided treatment is of interest after internal atrial cardioversion. AF duration, heart rate while the patient is taking drugs, ERP, and ERP relation to CL are independent predictors of AF recurrence. Maximised antiarrhythmic treatment following internal atrial cardioversion may benefit patients at high risk of AF recurrence.
Abbreviations
AF, atrial fibrillation
BCL, basal cycle length
CI, confidence interval
CL, cycle length
EP, electrophysiological study
ERP, effective refractory period
NYHA, New York Heart Association
SR, sinus rhythm
REFERENCES
- 1.Sopher SM, Murgatroyd FM, Slade AKD, et al. Low energy internal cardioversion of atrial fibrillation resistant to transthoracic shocks. Heart 1996;75:635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy S, Ricard P, Lau CP, et al. Multicenter low energy transvenous atrial defibrillation (XAD) trial results in different subsets of atrial fibrillation. J Am Coll Cardiol 1997;29:750–5. [DOI] [PubMed] [Google Scholar]
- 3.Juul-Moller S, Edvarsson N, Rehnquist-Ahlberg N. Sotalol versus quinidine for the maintenance of sinus rhythm after direct current conversion of atrial fibrillation. Circulation 1990;82:1932–9. [DOI] [PubMed] [Google Scholar]
- 4.Van Gelder IC, Crijns HJGM, Van Gilst WH, et al. Efficacy and safety of flecainide acetate in the maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation or atrial flutter. Am J Cardiol 1989;64:1317–21. [DOI] [PubMed] [Google Scholar]
- 5.Van Gelder IC, Crijns HJGM. Cardioversion of atrial fibrillation and subsequent maintenance of sinus rhythm. PACE 1997;20:2675–83. [DOI] [PubMed] [Google Scholar]
- 6.Tse HF, Lau CP, Ayers GM. Long-term outcome in patients with chronic atrial fibrillation after successful internal cardioversion. Am J Cardiol 1999;83:607–9. [DOI] [PubMed] [Google Scholar]
- 7.Wijffels MCEF, Kirchhof CJHJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 8.Crjins HJGM, Tjeerdsma G, de Kam PJ, et al. Prognostic value of the presence and development of atrial fibrillation in patients with advanced chronic heart failure. Eur Heart J 2000;21:1238–45. [DOI] [PubMed] [Google Scholar]
- 9.Boriani G, Biffi M, Capucci A, et al. Efficacy and tolerability in fully conscious patients of transvenous low-energy internal atrial cardioversion for atrial fibrillation. Am J Cardiol 1998;81:241–4. [DOI] [PubMed] [Google Scholar]
- 10.Boriani G, Biffi M, Pergolini F, et al. Low energy internal atrial cardioversion in atrial fibrillation lasting more than a year. PACE 1999;22:243–6. [DOI] [PubMed] [Google Scholar]
- 11.Roden DM. Role of the electrocardiogram in determining electrophysiologic end points of drug therapy. Am J Cardiol 1988;62:34H–8H. [DOI] [PubMed] [Google Scholar]
- 12.Morganroth J, Horowitz LN. Flecainide: its proarrhythmic effect and expected changes on the surface electrocardiogram. Am J Cardiol 1984;53:89B–94B. [DOI] [PubMed] [Google Scholar]
- 13.Hohnloser SH, Woosley RL. Sotalol. N Engl J Med 1994;331:31–8. [DOI] [PubMed] [Google Scholar]
- 14.Boriani G, Biffi M, De Simone N, et al. Repolarization changes in a double blind crossover study of dofetilide versus sotalol in the treatment of ventricular tachycardia. PACE 2000;23:1935–8. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Singh BN. Proarrhythmia with class III antiarrhythmic drugs: definition, electrphysiologic mechanisms, incidence, predisposing factors and clinical implications. J Cardiovasc Electrophysiol 1995;6:920–36. [DOI] [PubMed] [Google Scholar]
- 16.Hii JT, Wyse DG, Gillis AM, et al. Precordial QT interval dispersion as a marker of torsade de pointes: disparate effects of class Ia antiarrhythmic drugs and amiodarone. Circulation 1992;86:1376–82. [DOI] [PubMed] [Google Scholar]
- 17.Le Heuzey J, Boutjdir M, Gagey S, et al. Cellular aspects of atrial vulnerability. In: Attuel P, Olsson SB, Schlepper M, eds. The atrium in health and disease. Mount Kisco: Futura Publishing, 1989:81–94.
- 18.Attuel P, Childers RW, Haissaguerre M, et al. Failure in rate adaptation of the atrial refractory periods: new parameter to assess atrial vulnerability. PACE 1984;7:1382. [Google Scholar]
- 19.Manios EG, Kanoupakis EM, Chlouverakis GI, et al. Changes in atrial electrical properties following cardioversion of chronic atrial fibrillation: relation with recurrence. Cardiovasc Res 2000;47:244–53. [DOI] [PubMed] [Google Scholar]
- 20.Capucci A, Biffi M, Boriani G, et al. Dynamic electrophysiologic behaviour of human atria during paroxysmal atrial fibrillation. Circulation 1995;92:1193–202. [DOI] [PubMed] [Google Scholar]
- 21.Biffi M, Boriani G, Bronzetti G, et al. Electrophysiologic effects of flecainide and propafenone on atrial fibrillation cycle and relationship with arrhythmia termination. Heart 1999;82:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daoud EG, Bogun F, Harvey M, et al. Effect of atrial fibrillation on atrial refractoriness in humans. Circulation 1996,94:1600–6. [DOI] [PubMed] [Google Scholar]
- 23.Saksena S, Shankar A, Prakash A, et al. Catheter mapping of spontaneous and induced atrial fibrillation in man. J Intervent Card Electrophysiol 2000;4:21–8. [DOI] [PubMed] [Google Scholar]
- 24.Tse HF, Lau CP, Ayers GM. Heterogeneous changes in electrophysiologic properties in the paroxysmal and chronically fibrillating human atrium. J Cardiovasc Electrophysiol 1999;10:125–35. [DOI] [PubMed] [Google Scholar]
- 25.De Sisti A, Leclercq JF, Fiorello P, et al. Electrophysiologic characteristics of the atrium in sinus node dysfunction: atrial refractoriness and conduction. J Cardiovasc Electrophysiol 2000;11:30–3. [DOI] [PubMed] [Google Scholar]
- 26.Coumel P, Leclercq J. Role of the autonomic nervous system in paroxysmal atrial fibrillation. In: Touboul P, Waldo AL, eds. Atrial arrhythmias. Current concepts and managment. St Louis: Mosby Year Book, 1990:248–61.
- 27.Boriani G, Biffi M, Zannoli R, et al. Evaluation of atrial refractoriness and atrial fibrillation inducibility immediately after internal cardioversion in patients with chronic persistent atrial fibrillation. Cardiovasc Drugs Ther 1999;13:507–11. [DOI] [PubMed] [Google Scholar]
- 28.Fareth S, Benardeau A, Thibault B, et al. The T-type Ca++ channel blocker mibefradil prevents the development of a substrate for atrial fibrillation by tachycardia-induced atrial remodeling in dogs. Circulation 1999;100:2191–7. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Yu WC, Cheng JJ, et al. Effect of verapamil on long-term tachycardia-induced atrial electrical remodeling. Circulation 2000;101:202–6. [DOI] [PubMed] [Google Scholar]
- 30.Lesh MD, Calman JM, Roithinger FX, et al. Potential role of “hybrid therapy” for atrial fibrillation. Semin Intervent Cardiol 1997;2:267–71. [PubMed] [Google Scholar]
- 31.Krol RB, Saksena S, Prakash A. New devices and hybrid therapies for treatment of atrial fibrillation. J Intervent Card Electrophysiol 2000;4:163–9. [DOI] [PubMed] [Google Scholar]