Abstract
The recently cloned rat vanilloid receptor, VR1, can be activated by capsaicin, acid, and heat. To determine the molecular mechanisms facilitating channel opening in response to these stimuli, VR1 and six channels containing charge neutralization point mutations surrounding the putative channel pore domain were expressed and characterized in Xenopus laevis oocytes. Steady-state dose-response relationships, current-voltage relationships, ionic selectivities, and single-channel properties were recorded using voltage-clamp techniques. Three of the mutant channels are significantly more sensitive to capsaicin than is wild-type VR1, whereas none differed in their activation by acidic pH or temperature. Furthermore, one of the mutants has lost all positive cooperativity for capsaicin activation (Hill coefficient ≅ 1, VR1 ≅ 2), is much more selective for Ca2+, and exhibits a lower efficacy for acid than for capsaicin activation. Single-channel recordings show that capsaicin- and acid-activated channels have the same conductance, that the three mutants with increased capsaicin sensitivity exhibit higher open probabilities at submaximal capsaicin concentrations, and that the gating properties of capsaicin activation differ from those of acid activation. These data indicate that VR1 undergoes conformational changes upon capsaicin binding that it does not undergo in response to activation by protons or thermal stimuli. Furthermore, these structural rearrangements include the putative pore domain and reveal the location of an intracellular domain that contributes to the positive cooperativity seen for capsaicin activation.
Keywords: capsaicin, vanilloids, pain, pH, heat, VR1
Capsaicin, the primary pungent compound in “hot” chili peppers, produces pain and inflammation when placed on skin or mucus membranes. These responses are a consequence of capsaicin activating nonselective cation channel receptors within C and Aδ nociceptors and inducing the release of peptides and other transmitters from their peripheral and central terminals (1). Although a number of reports have demonstrated the existence of capsaicin receptors in sensory neurons (2, 3), the cloning of rat vanilloid receptor 1 (VR1) (4) was a breakthrough in understanding their properties and physiological functions (3, 5). VR1 subunits have now been identified in peripheral and central terminals of neurons in sensory ganglia (1, 3, 4, 6, 7). Electrophysiological studies of expressed VR1 receptors show that they are activated independently by capsaicin (EC50 = 0.7 μM), acid (pH50 = 5.4), and elevated temperatures ≥ 43°C (3, 6). These conditions are similar to those found in some physiological states (2, 3, 8). Further findings were that two or more of these activation pathways can act in concert to increase the sensitivity of VR1 receptors to any one stimulus (6, 8). To determine protein domains important for the activation of VR1 by capsaicin, protons, and heat, we constructed a number of VR1 point mutants and measured their responsiveness to these three activators. We found that mutations near the pore of VR1 channels can alter capsaicin activation properties, without changing proton and temperature activation properties. A preliminary report of these results has been published (9).
Materials and Methods
Site-Directed Mutagenesis and mRNA Synthesis.
The VR1 cDNA in the pcDNA3 vector (Invitrogen) was a generous gift from Dr. David Julius (UCSF) (4). Site-directed mutagenesis was performed using the QuikChange Kit (Stratagene). Base substitutions were confirmed by automated DNA sequencing, and RNA was prepared in the presence of cap analog with the Ambion Megascript Kit (Austin, TX).
Oocyte Electrophysiology: Two-Electrode Voltage Clamp.
Female Xenopus laevis were obtained from Nasco (Fort Atkinson, WI). Oocytes were surgically removed and defolliculated as described previously (10). Cells were voltage-clamped near the chloride equilibrium potential (−25 mV) to minimize the contribution of endogenous Ca2+-activated Cl− channels. To reduce Ca2+-dependent receptor desensitization (4), oocytes were bathed in a Ca2+-free buffer containing 1 mM MgCl2 and 0.1 mM BaCl2. For low pH experiments, the following buffers were used: Homopipes, pH 4.5–5.0; Mes, pH 5.0–6.5; and Hepes, pH 6.5–7.6. For experiments involving the relative efficacy of currents activated by protons and capsaicin, only one stimulus was applied to each oocyte. For experiments testing thermal responsiveness, the temperature of the recording solution in the buffers was controlled with an in-line SH-27A heater and a TC-324B temperature controller (Warner Instruments, Hamden, CT). For ion selectivity experiments holding potentials were changed in 10-mV steps in the presence and absence of 10 μM capsaicin, and background currents were subtracted. Extracellular solutions contained (in mM) either 120 Na-gluconate, 120 K-gluconate, 154.5 Mg-gluconate, or 182.4 Ca-gluconate, and 1 MgCl2, 10 Hepes (pH 7.6). Permeabilities relative to K+ were calculated (11), assuming bi-ionic conditions with an intracellular K+ concentration of 115 mM. For each condition experiments were repeated three to six times in separate oocytes.
Oocyte Electrophysiology: Patch Clamp.
Single channels were recorded using the patch-clamp method (12). Currents were amplified using an Axopatch 200 amplifier (Axon Instruments, Foster City, CA), low pass filtered at 2 kHz, and digitized at 10 kHz. For I/O recordings the bath solution consisted of (in mM) 105 K-gluconate, 5 Na-gluconate, 1 MgCl2, 0.5 EGTA, 0.1 EDTA, and 10 Hepes adjusted to pH 7.6. The pipette solution consisted of (in mM) 92 Na-gluconate, 2 KCl, 2 MgCl2, 1 EGTA, and 10 Hepes adjusted to pH 7.6. For investigations at pH 5.3, O/O patches were used. The bath solution contained 120 mM NaCl, 2 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 10 mM Mes. The pipette solution was the same, except that Hepes replaced Mes and the pH was 7.6.
Results
VR1 Activation by Capsaicin, Protons, and Thermal Energy.
To determine the role of the putative VR1 pore domain in the activation of this channel by capsaicin, protons, and heat, we prepared six VR1 point mutants (Fig. 1) and compared their functional properties to those of wild-type VR1 channels. The activation properties of channels in response to capsaicin or pH were determined using a two-pulse activation protocol. The data in Fig. 2 A and B show that two sequential applications of a maximal capsaicin concentration (10 μM) or pH change (pH 4.5) evoke large nondesensitizing currents of the same magnitude. This two-pulse protocol is thus used to construct dose-response data by normalizing the first (test) concentration of agonist to the second (maximal) agonist response. The activation time constants (0.9 ± 0.2 s, n = 6) are similar for the first two capsaicin applications, whereas deactivation time constants change, being approximately 25 s for the first pulse and slower (>35 s) for the second pulse. To reduce use-dependent changes in capsaicin and acid responsiveness, agonists were usually applied for the minimum time required for currents to reach steady state. After agonist application, a 5–10 min of washing permitted full recovery from either a pulse of 10 μM capsaicin or from a pulse of pH 4.5 buffer. Fig. 2B also shows that the kinetics of pH-mediated currents differ significantly from those produced by 10 μM capsaicin (Fig. 2A). Activation kinetics are biphasic, showing a large rapid component (time constant = 4.0 ± 0.8 s, n = 5) followed by small component with slower kinetics. Upon wash a transient inward current is evoked that precedes a rapid current deactivation (time constant = 3.2 ± 0.3 s, n = 6). Both the activation and deactivation kinetics are virtually identical for the first and second pulses of pH 4.5 buffer.
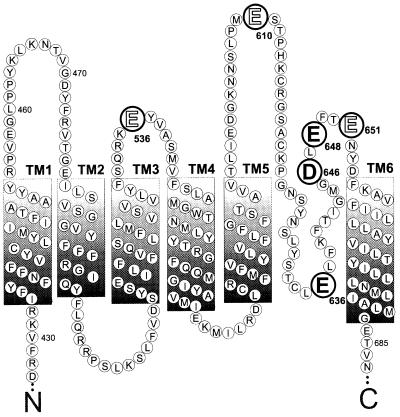
Figure 1.
Predicted transmembrane domains of VR1 highlighting amino acids that were mutated (large type). All mutations were D→N or E→Q. Residues whose mutation results in an altered capsaicin sensitivity are highlighted in bold. The N-terminal 426 amino acids and the C terminus from residues 688 to 838 are not shown.
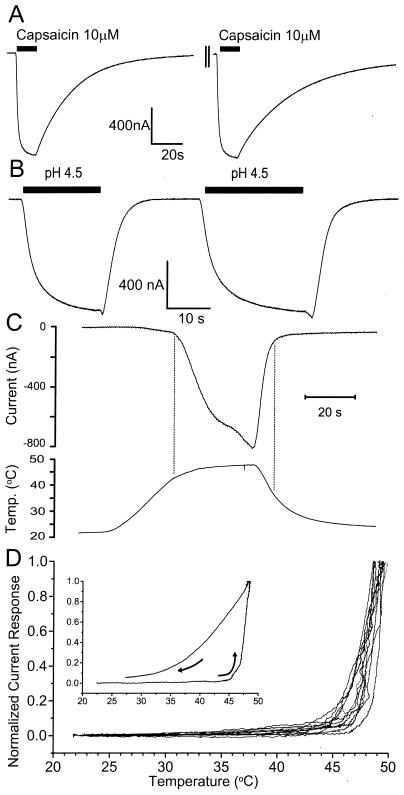
Figure 2.
Capsaicin, protons, and heat activate VR1 receptors. (A) Inward currents induced by two consecutive applications of the maximally effective dose (10 μM) of capsaicin. Capsaicin was applied to VR1-injected oocytes for 15 s (solid bar), perfused for 5–10 min (axis break marks), and reapplied for a 15-s pulse (solid bar). (B) Inward currents induced by two consecutive applications of maximally effective (pH 4.5) buffers. Solutions set to pH 4.5 were perfused for 15 s or 25 s (solid bars). (C) Upper trace: Current response of VR1 receptors to changes in temperature. Lower trace: Perfusate temperatures. The vertical lines indicate the threshold temperatures for VR1 channel activation and channel deactivation. (D) Representative plots of normalized current responses in response to increases in perfusate temperature. Temperature ramps from ≅22°C to 48 and 49°C in 12 oocytes injected with VR1 mRNA. Inward current magnitudes are normalized to peak currents. Vh = −25 mV.
The responses of VR1 channels to increases in temperature are shown in Fig. 2C. A temperature ramp from about 23°C to 48°C applied over a span of 60 s (lower trace) produces an inward current in VR1-injected oocytes (upper trace), but not in control oocytes (not shown). A large current increase is observed once the temperature reaches a threshold value, near 43°C (vertical line). Cooling results in a rapid current decrease which reaches baseline values at approximately 38°C. The dramatic current increase at the “threshold temperature” (Tth) is emphasized by replotting the heating data as normalized current, −(I (ToC)/Imax), vs. T (oC). Fig. 2D shows the plots of 12 independent experiments, showing that Tth = 43.8 ± 0.9°C. The threshold temperature we obtained for VR1 (43.8°C) is consistent with the value of 42–43°C obtained in HEK 293 cells and in Xenopus oocytes (4, 6, 13). Above Tth the current increases by e-fold approximately every 1.3°C, corresponding to a Q10 of 20.6, similar to sensory neurons (7, 8, 14). When the temperature of the perfusate was decreased from 48 to 25°C, the current's temperature dependence (Io) decreased to 5–8°C per e-fold change in current (Fig. 2D Inset).
Capsaicin Activation in VR1 Point Mutants.
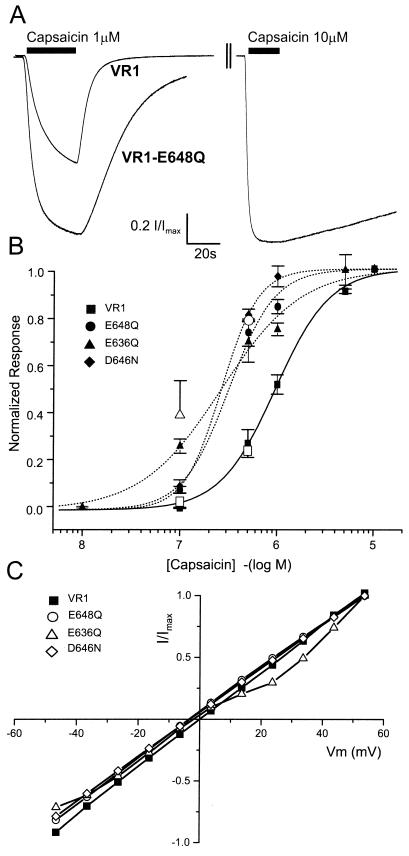
To determine the contribution of selected VR1 extracellular and pore-domain amino acids in the activation by capsaicin, protons, and temperature, we generated the following VR1 charge neutralization mutations (see Fig. 1): E536Q, E610Q, E636Q, D646N, E648Q, and E651Q. The E536Q mutation is presumably extracellular, located within the S3-S4 linker, and all of the other mutations are located near the putative pore region of VR1 (Fig. 1). The capsaicin dose-response data for VR1 and three mutants is shown in Fig. 3. Three of the six VR1 mutants tested, E636Q, D646N, and E648Q, have a 3-fold greater sensitivity to capsaicin than do VR1 channels (Fig. 3 A and B). Raw data records demonstrate that, whereas VR1 receptors are approximately half-activated by 1 μM capsaicin, VR1-E648Q receptors are nearly maximally activated (Fig. 3A). Dose-response data for E536Q, E610Q, and E651Q are not significantly different from those for VR1 (data not shown). The lines in Fig. 3B represent fits to the Hill equation resulting in the following EC50 values and Hill coefficients: VR1: 0.9 μM, 1.6; E648Q: 0.3 μM, 1.9; E636Q: 0.3 μM, 1.1; D646N: 0.3 μM, 2.3. For VR1, these results are in general agreement with previous studies (EC50 = 0.52–0.7 μM, 2.0–2.85) (4, 13, 15). One of the mutants, E636Q, exhibits a further novel phenotype in that the positive cooperativity observed in VR1 and the other point mutants is abolished.
Figure 3.
Responsiveness of VR1 and mutants to capsaicin. (A) Normalized current responses by the sequential application of 1 μM and 10 μM capsaicin to cells expressing either VR1 receptors or VR1-E648Q receptors. The interstimulus interval was always longer than 5 min. (B) Capsaicin dose-response data for VR1 and mutants. Each point represents the mean ± SEM for 2EVC data from at least four independent oocytes. The solid and dashed lines are optimized fits to the Hill equation. Open symbols indicate the relative Po values obtained from single-channel records of VR1 (□), E648Q (○), and E636Q (Δ). (C) Current-voltage relationship for VR1, E648Q, E636Q, and D646N. Steady-state currents were recorded at a series of voltages in the presence and absence of 10 μM capsaicin. Plots are normalized to maximal steady-state currents. Error bars (SEM) are omitted for the sake of clarity but were always less than 0.18I/Imax.
To determine whether any of these charge neutralization mutations near the pore altered the voltage dependence of channels, I/V relationships of capsaicin-activated currents were measured (Fig. 3C). With the exception of a small rectification found for the E636Q mutant, VR1 and the other mutants are ohmic over the voltage range measured.
Magnitude of Capsaicin and pH Activation of VR1 and Mutants.
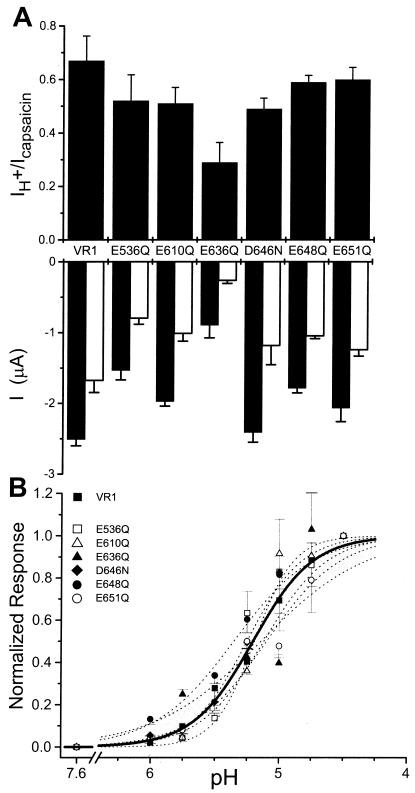
Fig. 4A compares maximal capsaicin-induced current responses (Lower, filled bars), with maximal pH-induced changes (Lower, open bars). The relative efficacy (IH+/Icapsaicin) is shown in the top panel. For all channel constructs, the maximal pH response is less than the maximal capsaicin response (Fig. 4A Lower). The relative efficacy of the currents for VR1 in oocytes was IH+/Icap ≅ 0.7. By comparison, in HEK 293 cells IH+/Icap ≅ 0.45 (13). Differences in raw current values are likely due to variability in protein expression because the single-channel properties of the mutants are similar to those of VR1 (Figs. 5 and 6). Fig. 4B shows the pH dose-response data for VR1 and the six channel constructs. For wild-type VR1 receptors pH50 = 5.2, and the Hill slope is 1.8, in agreement with previous experiments (6, 13). Best fits to the pH current-response curves for the six point mutants (n ≥ 8 independent oocytes) are not significantly different from the pH dependence of VR1 (Fig. 4B, P > 0.05).
Figure 4.
Efficacy and pH dose response of VR1 and mutants. (A) Relative efficacy of capsaicin and proton activation of VR1 and point mutants. Lower bars show the absolute steady-state current responses to 10 μM capsaicin (filled) or pH 4.5 buffer (open) measured from VR1 or mutant-expressing oocytes. Each oocyte was used for only one pulse of one type of activator (n = 5–6 per activator). Upper bars show the mean IH+/Icapsaicin ratio calculated by random pairing of capsaicin and proton trials. (B) Acid dose-response curves. Currents are normalized to the maximal response elicited by the pH 4.5 buffer. Each point represents the mean ± SEM currents obtained from at least four independent experiments. The lines shown are optimized fits to the Hill equation: ■, solid thick line, VR1; dashed lines, point mutations. Vh = −25 mV.
Figure 5.
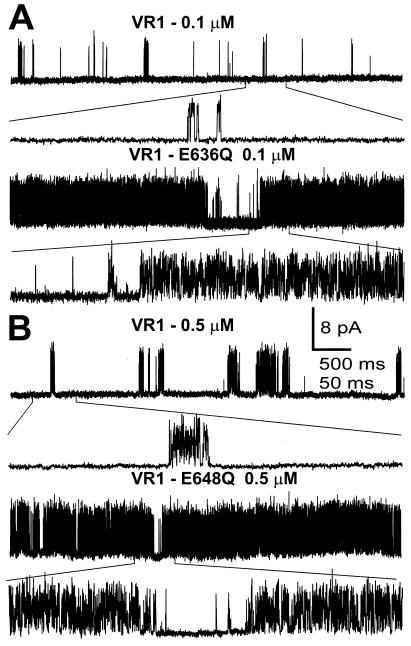
Single-channel recordings of VR1, E636Q, and E648Q activated by capsaicin. I/O patch-clamp records were obtained from oocytes expressing these receptors and exposed to the capsaicin concentrations shown. Channel gating using an expanded time scale is shown below each trace. (A) Representative traces of VR1 (upper) and E636Q (lower) activated by 0.1 μM capsaicin. (B) Representative traces of VR1 (upper) E648Q (lower) channels activated by 0.5 μM capsaicin. Vh = +80 mV.
Figure 6.
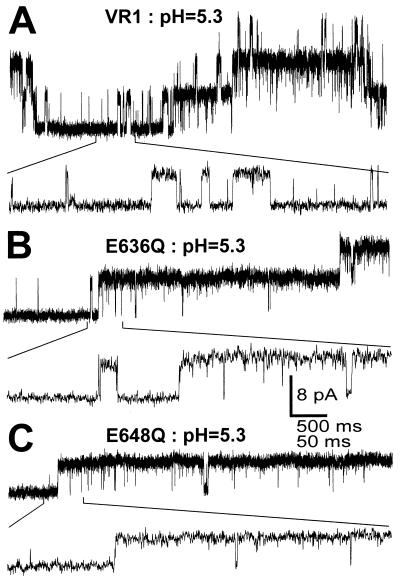
Single-channel recordings of VR1, E636Q, and E648Q activated by pH. O/O patch clamp records were obtained from oocytes expressing these receptors and exposed to pH 5.3 buffers. Channel gating using an expanded time scale is shown below each trace. (A) Representative traces of VR1 channels activated by pH 5.3. (B) Representative traces of VR1-E636Q receptors activated by pH 5.3. (C) Representative traces of VR1-E648Q receptors activated by pH 5.3. Vh = +80 mV.
Ion Selectivity of VR1 Mutants.
Table 1 compares the permeabilities of VR1 and the six VR1 mutants for K+, Na+, Mg2+, and Ca2+. For VR1, the relative permeabilities for K+, Na+, and Mg2+ are similar (≈1) and are about 2.6-fold less than that for Ca2+. Similar results were found in HEK 293 cells (4). None of the mutants exhibit significant selectivity differences between Na+ and K+. The largest permeability changes were found for the mutants E648Q and E636Q, which, compared with VR1, exhibit 3- and 7-fold higher Ca2+/K+ permeability ratios (7.9 and 17.6), respectively.
Table 1.
Ionic selectivity of VR1 and VR1 mutants
| Na+
|
K+
|
Mg2+
|
Ca2+
|
|||||
|---|---|---|---|---|---|---|---|---|
| Erev, mV | PNa+/PK+ | Erev, mV | PK+/PK+ | Erev, mV | PMg2+/PK+ | Erev, mV | PCa2+/PK+ | |
| VR1 | 4.2 ± 1.2 | 1.1 | 1.3 ± 1.6 | 1.0 | 17.9 ± 1.2 | 1.2 | 30.0 ± 3.9 | 2.6 |
| E648Q | −2.5 ± 0.8 | 0.90 | −1.5 ± 0.4 | 0.94 | 10.6 ± 0.6 | 0.71 | 44.0 ± 1.1 | 7.9 |
| E636Q | −1.2 ± 0.5 | 0.94 | −0.4 ± 0.9 | 0.97 | 15.4 ± 1.1 | 1.0 | 54.2 ± 1.5 | 17.6 |
| D646N | −2.8 ± 1.0 | 0.89 | −2.2 ± 0.6 | 0.90 | 10.9 ± 0.8 | 0.73 | 26.5 ± 1.1 | 2.0 |
Temperature Dependence of VR1 Mutants.
The elegant work of Tominaga et al. (6) revealed that VR1 becomes activated at the same temperature that elicits responses from a subset of small- and medium-diameter nociceptive neurons (3, 7, 16, 17). Surprisingly, our data indicate that none of these six charge neutralization mutants have a different temperature dependence (e-fold change in current per 1.3°C) or Tth [VR1: 43.8 ± 0.9°C (n = 12); E648Q: 43.5 ± 1°C (n = 8); E636Q: 43.2 ± 0.6°C (n = 9); D646N: 41.8 ± 0.9°C (n = 7)] from that of VR1 (Fig. 1D, data not shown).
Single-Channel Analysis of VR1 and Mutants.
The single-channel properties of VR1 and VR1 point mutations activated by either capsaicin (Fig. 5) or acidic solutions (Fig. 6) were measured using patch-clamp analysis. In the presence of capsaicin, VR1, VR1-E636Q, and VR1-E648Q were found to have the same fully open single-channel conductance of ≅100 pS at +80 mV (Fig. 5). The traces in Fig. 5 reveal the presence of very fast transitions between open and closed states for VR1, E636Q, and E648Q. The transition kinetics of the D646N mutant are similar (not shown). Indeed, most transitions do not reach the fully open state, as shown by all-points amplitude histograms (Fig. 7). The gating properties of VR1 channels activated by pH 5.3 are quite distinct from those of the capsaicin-activated channels (Fig. 6). Whereas the maximal conductance is similar to that observed in the presence of capsaicin, single-channel transitions remain in the open state for prolonged periods.
Figure 7.
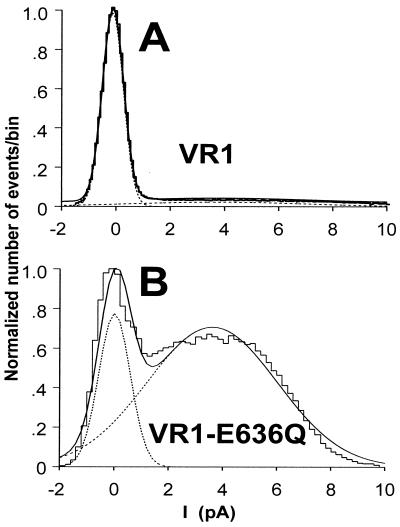
All-points amplitude histograms constructed from single-channel records of VR1 and VR1-E636Q in the presence of 0.1 μM capsaicin. (A) Current values from a single-channel VR1 trace at 0.1 μM were collected in 0.2-pA bins, and the resulting histogram was fit with a second-order Gaussian (dashed lines). The sum of these fits is shown as a smooth solid line. The area under each Gaussian fit was used to calculate Po for VR1 (0.03) and VR1-E636Q (0.56).
Mutants E636Q and E648Q were analyzed using all-points histogram analysis (Fig. 7) at 0.1 μM capsaicin for E636Q and 0.5 μM capsaicin for E648Q, because at these concentrations the mutants differ significantly from VR1 (see Fig. 3B). The mutants were also compared with VR1 at 10 μM, the maximal capsaicin concentration. The mean open probabilities (Po) are 0.32 (E636Q) and 0.03 (VR1) for 0.1 μM capsaicin and 0.56 (E648Q), compared with 0.23 (VR1) for 0.5 μM capsaicin (n = 4). At these two capsaicin concentrations, both mutants had significantly greater Po values than VR1 (P < 0.005). For 10 μM capsaicin, the mean Po values of the two mutants were not significantly different (Po = 0.78; E636Q and Po = 0.71; E648Q) from one another or from VR1 (Po = 0.70) (P > 0.4 in both cases; n = 3–8). These experiments show agreement between the open probabilities obtained from single-channel recordings compared with macroscopic currents obtained from two-electrode voltage-clamp studies (Fig. 3B), providing further evidence that the capsaicin sensitivity of E648Q is greater than it is for VR1. An additional finding is that in approximately 7% of all single-channel records (4/56 patch records), single channels with a conductance of only 50–75 pS were observed. This smaller conductance does not appear to be a filtering artifact, as the same conductance value was observed at 2-kHz and 5-kHz filter settings.
Discussion
Three distinct types of stimuli—capsaicin, acid, and heat—can induce pain and inflammation by activating polymodal nociceptive neurons. These neurons contain VR1 and VR1-like channels (4, 18), and there is good evidence that VR1 (or VR1 variants) can act as a molecular integrator for these irritating stimuli in sensory neurons, as well as in Xenopus oocytes expressing VR1 receptors (6, 19). The mechanisms by which these channels translate ligand binding, pH changes, and elevated temperatures into structural rearrangements leading to altered gating is not understood. To elucidate the molecular mechanisms leading from agonist binding altered gating, we have compared the activation properties evoked by capsaicin, pH, and temperature in VR1 and in six VR1 point mutants. Mutations were designed to neutralize glutamate (E) or aspartate (D) residues in or near the putative pore domain with glutamine (Q) or asparagine (N), which have previously been shown to alter pH activation in other ion channels (13, 20). Surprisingly, none of these mutations altered the pH dose-response or heat-response relationships; however, three mutations significantly altered the activation properties in response to capsaicin and changed the activation and deactivation kinetics. Hence, our data indicate that the protein domains involved in the gating of capsaicin can be modulated independently from the domains involved in the gating by protons or by heat.
Consistent with this model of independent activation pathways is data on dorsal root ganglion neurons showing that capsaicin activates VR1 channels only when it is applied to the intracellular side (21), whereas protons activate VR1 from the extracellular side (4). Further support for a model of separable activation pathways comes from the identification of VRL-1 receptors (18). This receptor/channel is homologous to VR1, but unlike VR1 it can only be activated by thermal stimuli and not by either capsaicin or protons. In single-channel recordings from intact neurons, channels have been identified that are activated by capsaicin but not by acid (pH 5.0) (7, 21) or by heat (50°C) (8). Finally, a recent study of VR1 mutants also demonstrates that pore mutations alter the IH+/Icapsaicin ratio (13). Taken together, these data imply that the vanilloid receptor family has independent gating mechanisms for capsaicin, protons, and heat.
It is of interest that the three point mutations with increased capsaicin sensitivity are in the putative pore domain (Fig. 1). By analogy to other putative six-transmembrane channels such as CNG and K+ channels, the mutated residues are likely to be located extracellularly (E648Q, D646N) or intracellularly (E636Q). Although we are unable to differentiate mutations at the as yet undefined capsaicin binding site(s) and mutations at “downstream” transduction domains, it appears improbable that all three mutations are at the binding site. Jung et al. (21) have shown that the capsaicin-binding site is likely on the intracellular side of the membrane, whereas two of the tested mutations are located extracellularly. Hence, we propose that a region of the pore and possibly the sixth transmembrane domain transduces capsaicin binding to channel gating. Such a model is consistent with structural rearrangements described during the proton-mediated activation of the Streptomyces K+ channel KCsA (22).
Our data reveal that in addition to altering the sensitivity to capsaicin of two of the putative pore mutants (Fig. 3), E636Q and E648Q have a much greater selectivity for Ca2+ than for monovalent cations (Table 1). Although VR1 channels are more selective for Ca2+ than for monovalent cations (PCa2+/PK+ = 2.9), for E636Q this ratio is 7-fold higher (Table 1). The PCa2+/PK+ ratio of 17.6 exceeds that for most ligand-gated channels and is similar to that of α7 nNAChRs (23). Hence the above residues localize the selectivity filter of VR1 to a region just upstream of the sixth transmembrane domain, in a position similar to that of voltage-gated channels, CNG channels, and the corresponding domains of KCsA channels (22). The finding that the E636Q mutation alters capsaicin dose-response parameters, relative efficacy, and selectivity indicates a reciprocal allosteric coupling between the capsaicin-binding site and the pore domain.
The single-channel analysis of VR1 and mutants shows that the increased sensitivity to capsaicin of the E636Q, D646N, and E648Q mutants revealed by macroscopic current analysis (Fig. 3) is observed as an increase in the Po (Fig. 5) and, specifically, as a decrease in the closed time component. Hence in the presence of capsaicin it appears that the closed state of the channel is stabilized to a greater extent than is VR1. The single-channel conductance of VR1 and all of the tested mutants is 90–100 pS at 80 mV, consistent with previously published values (4, 21, 24). In contrast, single-channel records of capsaicin-activated channels in dorsal root ganglion neurons yield conductance values of 46 pS (at 60 mV) (25) and 20 and 46 pS at 60 mV (8). Hence, at least some forms of capsaicin receptors in neurons differ significantly from VR1. It is likely that other VR1-like receptors remain to be identified, and/or that native receptors in neurons exist as heteromultimers.
In summary, we have identified three amino acid residues localized near the putative pore of VR1 receptors that contribute to the activation pathway for capsaicin, without altering the sensitivity to protons or thermal stimuli. These residues are located both intracellularly and extracellularly and indicate a structural rearrangement surrounding the pore during channel activation by capsaicin. Mutations at some of these sites change channel selectivity, highlighting a reciprocal relationship between the capsaicin-binding site and a pore domain containing the selectivity filter.
Acknowledgments
This work was supported by Grants DC01065 and NS31253 from the National Institutes of Health and a grant from the Philip Morris Corporation.
Abbreviation
- VR1
rat vanilloid receptor 1
- Homopipes
homopiperazine-N,N′-bis[2ethanesulfonic acid]
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230146497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230146497
References
- 1.Szallasi A, Blumberg P M. Pharmacol Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 2.Kress M, Zeilhofer M U. Trends Pharmacol Sci. 1999;20:112–118. doi: 10.1016/s0165-6147(99)01294-8. [DOI] [PubMed] [Google Scholar]
- 3.Caterina M J, Leffler A, Malmberg A B, Martin W J, Trafton J, Petersen-Zeitz K R, Koltzenberg M, Basbaum A I, Julius D. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Zygmunt P M, Petersson J, Anderson D A, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt E D. Nature (London) 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 6.Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilbert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Simon S A. Physiol Behav. 2000;69:363–378. doi: 10.1016/s0031-9384(00)00209-2. [DOI] [PubMed] [Google Scholar]
- 8.Nagy I, Rang H P. J Neurosci. 1999;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch J M, Erickson R P, Simon S A, Reinhart P H. Soc Neurosci. 1999;25:689. [Google Scholar]
- 10.DiCharia T J, Reinhart P H. J Physiol (London) 1995;489:403–418. doi: 10.1113/jphysiol.1995.sp021061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1993. [Google Scholar]
- 12.Hamill O P, Marty A, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Jordt S, Tominaga M, Julius D. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyklicky L, Vlachova V, Vitaskova Z, Dittert I, Kabat M, Orkland R K. J Physiol (London) 1999;517:181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunthorpe M J, Harries M H, Prinjha R K, Davis J B, Randall A. J Physiol (London) 2000;525.3:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy I, Rang H P. Neuroscience. 1999;88:995–997. doi: 10.1016/s0306-4522(98)00535-1. [DOI] [PubMed] [Google Scholar]
- 17.Treede R-D, Meyer R, Raja S N, Campbell J N. J Physiol (London) 1995;483:747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina M J, Rosen T A, Tominaga M, Brake A J, Julius D. Nature (London) 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 19.Guenter S, Kress M, Reeh P W. Eur J Neurosci. 1999;11:3143–3150. doi: 10.1046/j.1460-9568.1999.00734.x. [DOI] [PubMed] [Google Scholar]
- 20.Morrill J A, MacKinnon R. J Gen Physiol. 1999;114:71–83. doi: 10.1085/jgp.114.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J, Hwang S W, Kwak J, Lee S, Kang C, Kim W B, Kim D, Oh U. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perozo E, Cortes D M, Cuello L G. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- 23.Seguela P, Wadiche J, Dineley-Miller K, Dani J, Patrick J. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevan S, Szolcsanyi J. Trends Pharmacol Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 25.Lopshire J C, Nicol G D. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]