Abstract
Background: After acute myocardial infarction, the structural protein T is released considerably longer than cytosolic creatine kinase (CK), CK MB isoenzyme (CK-MB), or lactate dehydrogenase (LDH) and late troponin T release (> 48 hours after onset of chest pain) appears to be less affected by early coronary reperfusion.
Objective: To investigate the precision of a single measurement of circulating troponin T concentrations 72 hours after onset of chest pain compared with standard scintigraphic and enzymatic estimates of myocardial infarct size.
Methods: Quantitative single photon emission computed tomography thallium-201 scintigraphy at rest was performed in 37 patients 2–3 weeks after myocardial infarction (group 1: 14 patients without early coronary reperfusion; group 2: 23 patients with early reperfusion achieved by thrombolytic therapy, by percutaneous transluminal coronary angioplasty, or by both).
Results: In both groups, the number of myocardial segments with abnormal thallium-201 uptake indicating the individual extent of irreversible myocardial damage correlated significantly with the troponin T concentrations 72 hours after infarction as well as with peak concentrations of CK, CK-MB, and LDH.
Conclusion: The data show that a single measurement of circulating troponin T 72 hours after onset of chest pain—independent of reperfusion—is superior for the estimation of myocardial infarct size to measurement of peak CK, CK-MB, or LDH, which require serial determinations and depend on coronary reperfusion.
Keywords: acute myocardial infarction, infarct size, troponin T, creatine kinase, thallium-201 scintigraphy
After acute myocardial infarction (AMI), a patient's prognosis is closely related to the extent of irreversibly damaged myocardium.1–3 In routine clinical practice, infarct size is estimated non-invasively by electrocardiography, imaging procedures (such as myocardial radionuclide imaging and echocardiography), and serological tests. Each of these methods, however, has its limitations.
The value of serum concentrations of cytoplasmic enzymes such as creatine kinase (CK), its myocardial isoenzyme CK-MB, and lactate dehydrogenase (LDH) for estimation of the extent of myocardial cell damage is limited by the requirement for serial determinations to identify peak or cumulative serum concentrations. The latter depends strongly on variable normal serum concentrations and the effects of coronary reperfusion.4–6 Furthermore, specificity is relatively low since the expression of these enzymes is not restricted to the myocardium and increased serum concentrations can be observed as a result of other causes such as vigorous exercise and renal failure.7,8
In contrast to cytosolic markers, troponin T (TnT) is a structural protein of the contractile apparatus. After AMI, TnT is released continuously into the circulation for several days. TnT serum concentrations show a biphasic curve with one peak on the first day resulting from a release of the cytosolic TnT pool and a second “plateau” phase 3–4 days after the beginning of chest pain resulting from intramyocardial protein degradation.9–12 Compared with cytosolic markers, the second peak of TnT seems to be almost unaffected by early coronary reperfusion.13 In addition to its high sensitivity, TnT increase is also highly specific because TnT is expressed as skeletal isoforms and one highly cardiac specific isoform that is usually not detectable in patients without myocardial damage.14
Scintigraphic imaging with thallium-201, a potassium analogue, has a high sensitivity but lacks specificity because of its inability to distinguish between acute and prior infarction.15,16 In patients with a first myocardial infarction, however, thallium-201 scintigraphy is an accepted non-invasive method to estimate myocardial infarct size.
The aim of the present study was to investigate the reliability of TnT concentrations after 72 hours as a simple tool for the estimation of infarct size. For this purpose in patients with first AMI, myocardial infarct size was determined scintigraphically and then correlated with TnT serum concentrations 72 hours after the onset of symptoms, as well as peak and cumulative concentrations of CK, CK-MB, and LDH.
METHODS
Patients
Thirty seven consecutive patients with AMI were included. Exclusion criteria were chest pain lasting more than six hours at the time of admission and a history of prior myocardial infarction. Because of contraindications, no recanalisation was attempted in 10 patients and in an additional four patients recanalisation was not successful (group 1). Coronary recanalisation was achieved by intravenous thrombolysis, by percutaneous transluminal coronary angioplasty, or by both procedures in 23 patients (group 2). In all patients, coronary angiography was carried out either immediately after AMI or before being discharged from hospital.
Blood sampling
Blood samples were drawn every four hours during the first day, every eight hours on days 2 and 3, and then once daily until day 10. To allow clotting, the samples were kept at room temperature for 15 minutes and then centrifuged, and the serum was stored at −20°C until measurements were performed.
Cardiac enzyme measurements
CK and LDH activities were determined in a Chem 1 analyser (Technicon, Tarrytown, New York, USA) at 25°C, with reagents and protocols of the manufacturers. The upper limit of normal for total CK activity was 75 IU/l and for LDH 220 IU/l at 25°C.
The activity of the CK-MB isoenzyme was measured by an immunoinhibition assay (Boehringer Mannheim, Mannheim, Germany). The upper limit of normal was 10 IU/l or 6% of total CK activity, respectively.
Cardiac TnT was determined by a commercially available enzyme linked immunosorbent assay (ELISA) one step sandwich assay with streptavidin technology and two specific monoclonal antibodies developed by Katus and colleagues14 (Enzymun-Test system, Boehringer Mannheim).
The measuring range for TnT in this test is 0.1–15 ng/ml. TnT concentrations > 0.5 ng/ml were considered to indicate myocardial cell damage. Cross reactivity with skeletal muscle isoforms of TnT is < 0.5%.
Thallium-201 scintigraphy
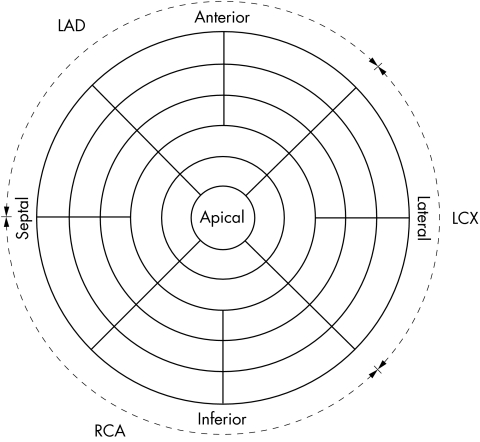
The extent of resting thallium-201 defects was determined by single photon emission computed tomography (SPECT). Ten to 18 days after myocardial infarction, each patient received 3.0 mCi thallium-201 intravenously at rest and was imaged 30 minutes later with a gamma camera (Siemens Orbiter ZLC 7500, Siemens AG, Erlangen, Germany) linked to a microcomputer (Elscint Microcomputer, Elscint, Wiesbaden, Germany). The camera was started from the right anterior oblique 30° projection and ended at the left posterior oblique 30° projection, collecting data from 32 views. Three dimensional polar maps were then displayed to evaluate the regional distribution of left ventricular thallium-201 activity (fig 1).17 The scintigraphic estimation of infarct size was defined as the number of left ventricular segments with thallium-201 activity < 67% of maximum.
Figure 1.
Polar map presentation of left ventricular thallium-201 uptake (33 segments).
Statistical analysis
The defect size in tomographic thallium-201 myocardial scintigrams was compared with serological parameters by linear regression analysis. To compare the correlation coefficients, the method of Hotelling18 was applied.
A probability value of p < 0.05 was considered significant. Continuous variables are shown as mean (SD).
RESULTS
Table 1 summarises the clinical data of the patients. Patients in the non-reperfusion (group 1) and reperfusion groups (group 2) were similar with regard to the infarcted coronary artery.
Table 1.
Clinical data of non-reperfusion (group 1) and reperfusion (group 2) groups of patients
| Group 1 (n=14) | Group 2 (n=23) | |
| Age (years) | 65 (11) | 60 (12) |
| Male sex | 9 (64%) | 17 (74%) |
| Infarct related vessel | ||
| RCA | 9 (64%) | 13 (57%) |
| LAD | 3 (22%) | 6 (26%) |
| LCX | 2 (14%) | 4 (17%) |
Data are mean (SD) or frequency.
LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery.
Mean age was non-significantly higher in group I than in group II (NS).
Serological estimation of infarct size
Peak and cumulative serum concentrations of TnT, CK, CK-MB, and LDH were obtained by serial determinations throughout the hospital stay. Table 2 shows individual data.
Table 2.
Scintigraphic and serological data
| Patient | Infarct related vessel | Treatment | No of segments with <67% thallium-201 activity | TnT at 72 hours (ng/ml) | CKmax (IU/l) | CK-MBmax (IU/l) | LDHmax (IU/l) |
| Group 1 | |||||||
| 1 | RCA | Conservative | 6 | 5.2 | 240 | 37 | 445 |
| 2 | RCA | Conservative | 5 | 4.2 | 273 | 40 | 323 |
| 3 | LAD | Conservative | 12 | 8.6 | 2031 | 215 | 1192 |
| 4 | RCA | Conservative | 6 | 14.2 | 1062 | 128 | 815 |
| 5 | LAD | Conservative | 15 | 15.4 | 978 | 84 | 997 |
| 6 | RCA | Conservative | 8 | 8.8 | 1700 | 190 | 1454 |
| 7 | LCX | Conservative | 5 | 3 | 210 | 19 | 384 |
| 8 | RCA | Conservative | 5 | 3 | 768 | 51 | 658 |
| 9 | LCX | Conservative | 9 | 9.8 | 1806 | 175 | 1218 |
| 10 | RCA | Conservative | 8 | 3.5 | 403 | 46 | 440 |
| 11 | RCA | Unsuccessful PTCA | 4 | 3.9 | 431 | 71 | 453 |
| 12 | RCA | Unsuccessful PTCA | 11 | 19 | 1116 | 114 | 791 |
| 13 | LAD | Unsuccessful Sk lysis | 1 | 1.8 | 245 | 24 | 325 |
| 14 | RCA | Unsuccessful Sk lysis | 1 | 4.6 | 589 | 61 | 332 |
| Mean (SD) | 6.9 (4.0) | 7.5 (5.4) | 847 (626) | 90 (65) | 702 (383) | ||
| Group 2 | |||||||
| 1 | RCA | PTCA | 2 | 1.7 | 617 | 50 | 358 |
| 2 | RCA | rtPA lysis | 5 | 3.7 | 812 | 72 | 682 |
| 3 | RCA | Sk lysis | 9 | 8 | 855 | 114 | 761 |
| 4 | RCA | rtPA lysis | 6 | 5.3 | 998 | 63 | 462 |
| 5 | LCX | Sk lysis | 5 | 2.1 | 534 | 22 | 340 |
| 6 | RCA | Sk lysis | 8 | 6.8 | 1510 | 200 | 91 |
| 7 | RCA | Sk lysis | 8 | 6.4 | 1228 | 123 | 1086 |
| 8 | LAD | Sk lysis | 4 | 2.9 | 541 | 60 | 312 |
| 9 | LCX | Sk lysis | 4 | 6.2 | 920 | 96 | 626 |
| 10 | LAD | rtPA lysis | 7 | 6.8 | 1144 | 86 | 685 |
| 11 | RCA | Sk lysis | 9 | 6.9 | 643 | 78 | 601 |
| 12 | RCA | rtPA lysis | 11 | 7.3 | 1354 | 77 | 840 |
| 13 | RCA | Sk lysis | 1 | 3.0 | 670 | 61 | 405 |
| 14 | RCA | rtPA lysis | 10 | 11.0 | 1938 | 160 | 1190 |
| 15 | LCX | rtPA lysis | 5 | 1.5 | 253 | 25 | 224 |
| 16 | LCX | rtPA lysis + PTCA | 11 | 6.7 | 1480 | 144 | 863 |
| 17 | LAD | Sk lysis | 14 | 18.5 | 2045 | 189 | 1166 |
| 18 | RCA | Sk lysis | 2 | 6.1 | 780 | 72 | 456 |
| 19 | RCA | Sk lysis | 7 | 3.6 | 549 | 37 | 454 |
| 20 | LAD | PTCA | 1 | 2.6 | 668 | 74 | 518 |
| 21 | LAD | rtPA lysis | 3 | 4.1 | 837 | 55 | 501 |
| 22 | LAD | Sk lysis | 3 | 3.7 | 667 | 24 | 441 |
| 23 | RCA | Sk lysis | 6 | 3.2 | 904 | 67 | 731 |
| Mean (SD) | 6.1 (3.5) | 5.6 (3.7) | 954.2 (454.9) | 84.7 (49.5) | 635.1 (273.5) |
CKmax, peak concentration of creatine kinase; CK-MBmax, peak concentradion of creatine kinase MS isoenzyme; LDHmax, peak concentration of lactate dehydrogenase; PTCA, percutaneous transluminal coronary angioplasty; rtPA lysis, alteplase lysis; Sk-lysis, streptokinase lysis; TnT, troponin T.
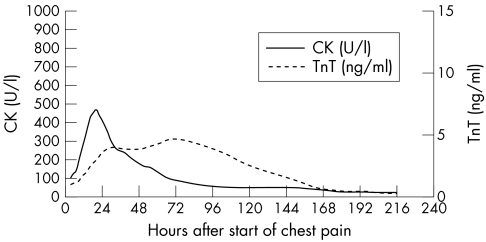
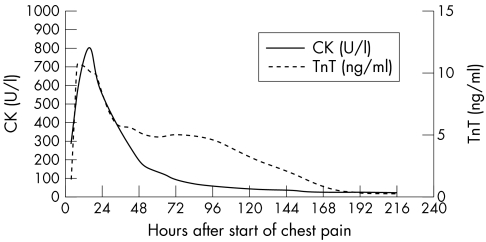
In both groups, TnT showed a biphasic curve with a brief peak within the first 24 hours and a second peak on the third to fourth day after the onset of chest pain. The first peak obtained in group 1 was lower than the second peak, whereas in group 2 the first peak clearly exceeded the second peak (fig 2, fig 3).
Figure 2.
Time course of troponin T (TnT) and creatine kinase (CK) release in group 1 (non-reperfusion group, n = 14).
Figure 3.
Time course of TnT and CK release in group 2 (reperfusion group, n = 23).
Thallium-201 imaging
“Scintigraphic infarct size” varied between 1 and 15 left ventricular segments with thallium-201 activity < 67% in group 1 (3–45%, mean 21%) and between 1 and 14 segments in group 2 (3–42%, mean 18%).
Correlation between scintigraphic estimation of infarct size and peak serum concentrations of cardiac markers
A significant linear correlation was obtained between scintigraphic infarct size and the peak serum concentrations of TnT, CK, CK-MB, and LDH (table 3).
Table 3.
Correlation coefficients between scintigraphic and serological determination of infarct size at peak concentrations of cardiac markers
| Group 1 (n=14) | Group 2 (n=23) | |||
| Marker | r | p Value | r | p Value |
| TnT at 72 hours | 0.718 | <0.004 | 0.773 | <0.001 |
| CKmax | 0.610 | <0.021 | 0.737 | <0.001 |
| CK-MBmax | 0.557 | <0.039 | 0.669 | <0.001 |
| LDHmax | 0.685 | <0.007 | 0.765 | <0.001 |
TnT was measured 72 hours after onset of chest pain.
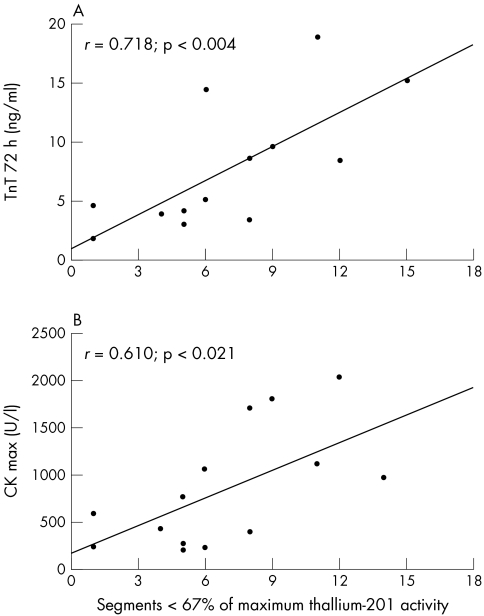
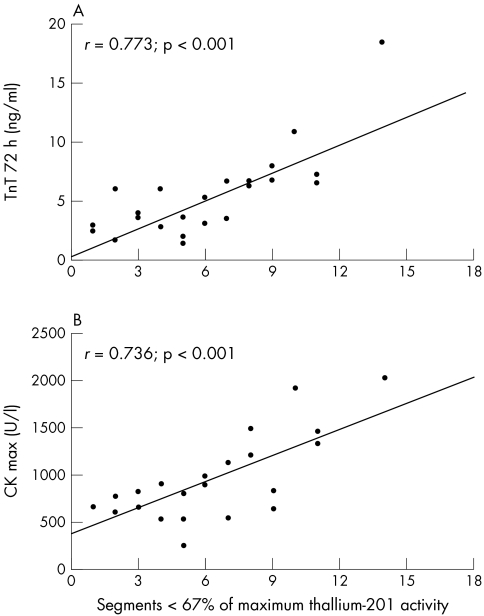
Compared with cytosolic markers such as CK, CK-MB, and LDH, the TnT serum concentrations determined 72 hours after onset of chest pain reached the highest correlation coefficients with the scintigraphically estimated infarct size in both groups. Figures 4 and 5 show the correlation between scintigraphic and serological estimates of infarct size by TnT and CK.
Figure 4.
Correlation (A) between scintigraphic infarct size and TnT serum concentrations 72 hours after the onset of symptoms, and (B) between scintigraphic infarct size and maximal CK serum concentrations in group 1 (n = 14).
Figure 5.
Correlation (A) between scintigraphic infarct size and TnT serum concentrations 72 hours after the onset of symptoms, and (B) between scintigraphic infarct size and maximal CK serum concentrations in group 2 (n = 23).
Correlation between scintigraphic estimation of infarct size and cumulative serum concentrations of cardiac markers
Like the peak serum concentrations, the cumulative concentrations of all cardiac markers determined in this study correlated significantly with the scintigraphic infarct size, independent of coronary reperfusion (table 4).
Table 4.
Correlation coefficients between scintigraphic and serological determination of infarct size at cumulative (cum) concentrations of cardiac markers
| Group 1 (n=14) | Group 2 (n=23) | |||
| Marker | r | p Value | r | p Value |
| TnTcum | 0.819 | <0.001 | 0.780 | <0.001 |
| CKcum | 0.655 | <0.011 | 0.621 | <0.003 |
| CK-MBcum | 0.623 | <0.017 | 0.585 | <0.003 |
| LDHcum | 0.603 | <0.029 | 0.746 | <0.001 |
Correlation between cumulative CK concentration and the cumulative serum concentration of other cardiac markers
The serological estimation of infarct size, determined by the cumulative concentration of CK, correlated significantly with the cumulative serum concentrations of TnT, CK-MB, and LDH (table 5).
Table 5.
Correlation coefficients between cumulative CK concentration and cumulative concentrations of TnT, CK-MB, and LDH
| Group 1 (n=14) | Group 2 (n=23) | |||
| Marker | r | p Value | r | p Value |
| TnTcum | 0.684 | <0.008 | 0.698 | <0.001 |
| CK-MBcum | 0.943 | <0.001 | 0.751 | <0.001 |
| LDHcum | 0.856 | <0.001 | 0.667 | <0.001 |
DISCUSSION
Data obtained in the present study show that scintigraphic versus serological estimation of infarct size is significantly correlated for each serum marker tested, independent of coronary reperfusion. The highest correlation coefficients were achieved with serum concentrations of TnT determined 72 hours after the onset of chest pain and with its cumulative concentrations. The correlation coefficients for the cumulative concentrations of CK and the other cytosolic enzymes were slightly better than for cumulative CK and cumulative TnT. This may be explained by the different release kinetics of the cytosolic enzymes and the properties of TnT as a structural protein. Estimation of infarct size, however, is more precise with cumulative TnT concentrations than with cumulative cytosolic enzyme concentrations, according to the correlation coefficient between scintigraphic estimation of infarct size and cumulative concentrations of marker proteins (table 4).
As serological markers for the estimation of infarct size, serum concentrations of CK, CK-MB, and LDH are mostly used. The accuracy of this approach, however, is adversely affected by several factors: The normal range of serum enzyme activity ranges from 10–80 IU/l for CK, < 10 IU/l for CK-MB, and 140–290 IU/l for LDH. Compared with this normal variation, the serum concentration increase of these cytosolic enzymes after AMI is relatively low. For example, CK and LDH activities increase < 60-fold and < 10-fold, respectively.19–21 Another disadvantage of cytosolic enzymes is their short persistence in the serum after AMI. CK and LDH serum concentrations decrease to normal concentrations within 3–6 days and 6–15 days, respectively.22–25 Furthermore, these cytosolic enzymes are also present in non-cardiac muscular tissues and can therefore be increased for other reasons, independent of myocardial cell damage, such as renal failure, myopathies, trauma, or even after vigorous exercise or in chronic alcoholism.7,8,26
In contrast to cytosolic enzymes, the baseline concentration of TnT is below the detection limit of commercially available assays but may exceed 400 times the discriminating serum concentration in myocardial cell damage.27 Compared with the cytosolic markers, TnT serum concentrations increase slightly earlier after the onset of symptoms at about 3.5 hours versus 4–8 hours (CK) and 6–12 hours (LDH).21 Since cross reactivity with skeletal TnT is < 1%, the specificity and sensitivity of TnT are about 96% and 100%, respectively.14
Because of the release kinetics of cytosolic enzymes, which reach a single, brief maximal peak within the first 24–48 hours after AMI and decrease to normal concentrations rapidly thereafter, serial blood sampling is mandatory to obtain the maximal or cumulative serum concentrations for estimation of infarct size. Prolonged TnT release over several days with a second peak on the third to fourth day allows a more precise estimation of infarct size over a longer period of time, even with a delay of several days after the onset of symptoms. When comparing the study groups in terms of peak serum CK concentration and infarct size estimated by scintigraphy (fig 4B, fig 5B), the gradient of the curve was greater in the reperfusion group than in the non-reperfusion group, reflecting a superincrease of maximal CK in the case of coronary reperfusion. In contrast with CK, the curve of TnT 72 hours after the onset of chest pain is not steeper in the reperfusion subgroup than in the non-reperfusion group (fig 4A, 5A); therefore, TnT 72 hours after the onset of chest pain seems to be almost unaffected by coronary reperfusion. The present study shows that a single blood sample 72 hours after the onset of symptoms allows a rather precise estimation of infarct size with serum TnT when compared with scintigraphic estimation. This result is supported by the findings of Kragten and colleagues.28 In 22 patients with AMI, these authors also showed that the cumulative as well as the second TnT peak concentration correlated significantly with the cumulative release of cytoplasmic enzyme in reperfused myocardial infarctions; however, their results were not related to infarct size.28
Study limitations
In addition to the methodological considerations mentioned above, further limitations have to be taken into account. Using thallium-201 SPECT imaging, assessment of infarct size can be improved by correcting for variable left ventricular mass in the individual segments. Also, the differing absorption in anterior and posterior myocardial segments was not corrected.
Conclusions
According to the present results, a single measurement of circulating TnT 72 hours after the onset of symptoms is a simple and reliable tool for serological estimation of myocardial infarct size independent of early coronary reperfusion.
Abbreviations
CK, creatine kinase
CK-MB, creatine kinase MB isoenzyme
ELISA, enzyme linked immunosorbent assay
LDH, lactate dehydrogenase
SPECT, single photon emission computed tomography
TnT, troponin T
REFERENCES
- 1.Geltman EM, Ehanni AA, Campbell MK, et al. The influence of location and extent of myocardial infarction on long-term ventricular dysrhythmia and mortality. Circulation 1979;60:805–14. [DOI] [PubMed] [Google Scholar]
- 2.Chapman BL. Correlation of mortality rate and serum enzymes in myocardial infarction. Br Heart J 1971;33:643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts R, Husain A, Ambos HD, et al. Relation between infarct size and ventricular arrhythmia. Br Heart J 1975;37:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katus HA, Diedrich KW, Schwarz F, et al. Influence of reperfusion on serum concentrations of cytosolic creatine kinase and structural myosin light chains in acute myocardial infarction. Am J Cardiol 1987;60:440–5. [DOI] [PubMed] [Google Scholar]
- 5.Katus HA, Scheffold T, Bode C. Der Myokardinfarkt: akute Intervention. Z Kardiol 1993;82:59–70. [PubMed] [Google Scholar]
- 6.Roberts R. Enzymatic estimation of infarct size. Thrombolysis induced its demise: will it now rekindle its renaissance? Circulation 1990;81:707–10. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe AS, Garfinkel BT, Ritter CS, et al. Plasma MB creatine kinase after vigorous exercise in professional athletes. Am J Cardiol 1984;53:856–8. [DOI] [PubMed] [Google Scholar]
- 8.Mair J. Cardiac troponin I and troponin T: are enzymes still relevant as cardiac markers? Clin Chim Acta 1997;257:99–115. [DOI] [PubMed] [Google Scholar]
- 9.Katus HA, Scheffold T, Remppis A, et al. Proteins of the troponin complex. Lab Med 1992;23:311–7. [Google Scholar]
- 10.Remppis A, Richardt G, Scheffold T. Troponin T: intrazelluläre Kompartimentierung und Freisetzungskinetik. Z Kardiol 1991;80:72. [Google Scholar]
- 11.Remppis A, Scheffold T, Greten J, et al. Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol 1995;27:793–803. [DOI] [PubMed] [Google Scholar]
- 12.Lavin F, Kane M, Forde A, et al. Comparison of five cardiac markers in the detection of reperfusion after thrombolysis in acute myocardial infarction. Br Heart J 1995;73:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katus HA, Remppis A, Scheffold TH, et al. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 1991;67:1360–7. [DOI] [PubMed] [Google Scholar]
- 14.Katus HA, Looser S, Hallermayer K, et al. Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem 1992;38:386–93. [PubMed] [Google Scholar]
- 15.Wackers FJ, Sokole EB, Samson G, et al. Value and limitations of thallium-201 scintigraphy in the acute phase of myocardial infarction. N Engl J Med 1976;295:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Morrison J, Coromilas J, Munsey D, et al. Correlation of radionuclide estimates of myocardial infarction size and release of creatine kinase-MB in man. Circulation 1980;62:277–87. [DOI] [PubMed] [Google Scholar]
- 17.Stirner H, Büll U, Kleinhans E. Three-dimensional ROI-based quantification of stress/rest Tl-201 myocardial SPECT: presentation of method. Nucl Med 1986;25:128–33. [PubMed] [Google Scholar]
- 18.Sachs L. Angewandte Statistik. Berlin: Springer, 1984.
- 19.Sundermann A. Current concepts of “normal values”, “reference values” and “discrimination values” in clinical chemistry. Clin Chem 1975;21:1873–7. [PubMed] [Google Scholar]
- 20.Shell WE, DeWood MA, Klingerman M, et al. Early appearance of MB-creatine kinase activity in non-transmural myocardial infarction detected by a sensitive assay for the isoenzyme. Am J Med 1981;71:254–62. [DOI] [PubMed] [Google Scholar]
- 21.Katus HA, Remppis A, Neumann FJ, et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation 1991;83:902–12. [DOI] [PubMed] [Google Scholar]
- 22.Smith A. Enzymes and routine diagnosis. In: Hearse DJ, De Leiris J, eds. Enzymes in cardiology: diagnosis and research. New York: John Wiley and Sons, 1979:200–46.
- 23.Irvin RG, Cobb FR, Roe CR. Acute myocardial infarction and MB creatine phosphokinase: relationship between onset of symptoms of infarction and appearance and disappearance of enzyme. Arch Intern Med 1980;140:329–34. [PubMed] [Google Scholar]
- 24.Ljungdahl L, Gerhardt W, Hofvendahl S. Serum creatine kinase B subunit activity in diagnosis of acute myocardial infarction. Br Heart J 1980;43:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction: recommendations based on a quantitative analysis. Ann Intern Med 1986;105:221–33. [DOI] [PubMed] [Google Scholar]
- 26.Nygren A. Serum creatine phosphokinase in chronic alcoholism. Acta Med Scand 1967;182:383–7. [DOI] [PubMed] [Google Scholar]
- 27.Dengler TJ, Zimmermann R, Braun K, et al. Elevated serum concentrations of cardiac troponin T in acute allograft rejection after human heart transplantation. J Am Coll Cardiol 1998;32:405–12. [DOI] [PubMed] [Google Scholar]
- 28.Kragten JA, Hermens WT, Dieijen-Visser MP. Cardiac troponin T release into plasma after acute myocardial infarction: only fractional recovery compared with enzymes. Ann Clin Biochem 1996;33:314–23. [DOI] [PubMed] [Google Scholar]