Familial Mediterranean fever (FMF) is an autosomal recessively inherited inflammatory disease that primarily affects Jews, Armenians, Turks, and Arabs. It is characterised by recurrent self limited attacks of fever accompanied by inflammation of the peritoneum, synovium, and pleura. The gene responsible for FMF (MEFV) has been cloned recently on the short arm of chromosome 16 and more than 20 mutations have been identified.1 The gene product pyrin (marenostrin) is thought to control the inflammatory response by regulating the expression of some target genes that are involved in the suppression of inflammation. Thus, it was suggested that mutations in the MEFV gene prevent the normal pyrin (marenostrin) mediated negative feedback mechanism and trigger inflammation. Recently several studies suggested that having a MEFV mutation might act as an additional susceptibility factor in inflammatory conditions.2
Rheumatic heart disease (RHD) is a major sequel of rheumatic fever (RF), which is also an inflammatory disease that occurs after streptococcal throat infections. The role of genetic factors in the pathogenesis of RF is not clearly known. Familial predisposition to acquiring RF has been appreciated for a long time. Previous studies clearly demonstrated that children who developed RF had an exaggerated antibody response after streptococcal pharyngeal infections. These studies indicated that patients with RF might be genetically programmed to respond abnormally to streptococcal infections.3 Previous work of Eliakim and colleagues4 and a recent study by our group5 showed increased prevalence of RHD in patients with FMF. This relation suggested that individuals having MEFV mutations might be more prone to the late complications of streptococcal infections such as RF. In order to test this hypothesis mutation analysis for MEFV was carried out in an unselected group of patients with RHD without signs and symptoms of FMF.
METHODS
Twenty seven Turkish patients (15 female, 12 male) with RHD were enrolled in the study. Patients' ages ranged between 7–18 years (mean (SD) 12.7 (2.8) years). Diagnosis of RF was based on Jones' criteria updated in 1992. None of the patients had the diagnosis of FMF. The valvar lesion in all but two patients was mitral regurgitation. Eleven patients had aortic regurgitation as well. Detailed family histories for FMF, RF, and RHD were recorded. Written informed consents for enrolment were obtained from all participants before the study. Fifty four chromosomes from patients were analysed. The two hot spots (exons 10 and 2) for MEFV mutations were investigated by sequence analysis.
RESULTS
Seven MEFV mutations were found in 54 chromosomes (gene frequency 1:7.7). The prevalence of FMF has been reported as 0.93:1000 in Turkey.6 Thus, the estimated gene frequency was calculated as 1:32.7 according to the following formula:
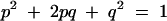 |
where p = frequency of normal allele; p2 = frequency of normal phenotype; q = frequency of the FMF allele; q2 = frequency of the FMF phenotype; and 2pq = proportion of carriers.
We compared the gene frequency for MEFV mutations of patients with RHD (1:7.7) with the estimated gene frequency of Turkish children (1:32.7). We found approximately four times greater MEFV mutation prevalence in patients with RHD than in the normal population in Turkey. Six out of 27 patients were found positive for one of the sequenced MEFV mutations (table 1). All but one of the patients had only one MEFV mutation. A 14 year old boy with mitral and aortic regurgitation had both M680I and V726A mutations but he had no symptoms suggesting FMF, and no family history for FMF. None of the patients had a family history for FMF. However, eight out of 27 patients (30%) had at least one relative with RF or RHD.
Table 1.
Clinical features of six patients with rheumatic heart disease bearing MEFV mutations
| MEFV mutation | Age (years)/ sex | Valvar lesion |
| M680I, V726A | 14/male | MR+AR |
| M680I | 14/female | MR |
| M680I | 18/female | MR |
| M680I | 12/female | MR |
| M694V | 10/female | MR+AR |
| M694V | 13/female | MR+AR |
MR, mitral regurgitation; AR, aortic regurgitation.
DISCUSSION
The genetic defect in FMF is proposed to be the impaired control of inflammation in response to certain recognised or unrecognised stimuli.1 Indeed, chronic inflammatory disorders such as vasculitides and Behçet's disease were reported to be common in patients with FMF.2
Estimated MEFV mutation frequency in Turks was calculated as 1:32.7. It was reported that the strikingly high gene frequencies for FMF in Mediterranean populations could be explained by the hypothesis of heterozygote selection. Heterozygotes might have a survival advantage by exhibiting greater but controlled inflammatory responses to a specific pathogen or class of pathogens.1 Although these enhanced inflammatory responses could be useful in the eradication of the pathogen(s), they might trigger the inflammation cascade resulting non-suppurative inflammatory diseases in some genetically susceptible individuals. Streptococci may act as a responsible pathogen in this scenario. Inflammatory disorders that might be related to streptococci, such as polyarteritis nodosa and Henoch-Schönlein purpura, are encountered in FMF more commonly than in the general population.2 In addition acute poststreptococcal glomerulonephritis has been characterised as a type of renal involvement,5 and RHD has been reported4,5 to be more frequent in patients with FMF than in the normal population. Moreover, increased antistreptolysin O titres have been reported in FMF patients.4 This is the proof of greater antibody response against streptococci in patients with FMF. Exaggerated antistreptococcal antibody response is also a characteristic feature of RF. Although RF can follow any group A β haemolytic streptococcus pharyngitis, it was demonstrated that children who developed RF had an exaggerated antibody response to streptococcal antigens when they were compared with children who had recovered from a streptococcal upper respiratory tract infection.3
Increased RF or RHD incidence in relatives of our patients strongly supports previous evidence suggesting a familial predisposition in RF. There is a strong consensus that RF represents an abnormal host immune response in genetically predisposed individuals to those streptococcal antigens cross reactive with heart, brain, and joint tissues.3 Considering the basic defect of FMF, which we propose to be the impaired control of the immune response, we speculate that even bearing only one MEFV mutation might directly or indirectly result in immune hyperreactivity against streptococcal antigens that leads to development of RF. Although a predisposition to RF is more likely to be multifactorial in origin, or may be polygenic, this is the first study implicating the possible role of the MEFV gene in the pathogenesis of RF.
Abbreviations
FMF, familial Mediterranean fever
MEFV, familial Mediterranean fever gene
RF, rheumatic fever
RHD, rheumatic heart disease
REFERENCES
- 1.The International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell 1997;90:797–807. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz T, Langevitz P, Zemer D, et al. Behcet's disease in familial Mediterranean fever: characterization of the association between the two diseases. Semin Arthritis Rheum 2000;29:286–95. [DOI] [PubMed] [Google Scholar]
- 3.Veasy G, Hill HR. Immunologic and clinical correlations in rheumatic fever and rheumatic heart disease. Pediatric Infec Dis J 1997;16:400–7. [DOI] [PubMed] [Google Scholar]
- 4.Eliakim M, Levy M, Ehrenfeld M, eds. Recurrent polyserositis. Amsterdam: Elsevier North-Holland Press, 1981:32–4.
- 5.Tekin M, Yalçýnkaya F, Tümer N, et al. Familial Mediterranean fever and acute rheumatic fever: a pathogenetic relationship? Clin Rheumatol 1999;18:446–9. [DOI] [PubMed] [Google Scholar]
- 6.Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol 1998;25:2445–9. [PubMed] [Google Scholar]


