It is 40 years since Starr and Edwards' description of successful prosthetic valve replacement in 1961. Some patients who underwent valve replacement with the original Starr-Edwards prosthesis in the 1960s are alive to this day. The Starr-Edwards ball and cage prosthesis, albeit in modified form, is still available commercially. Each year more than 6000 patients in the UK and 60 000 in the USA alone undergo valve replacement surgery. In the last 40 years more than 80 models of prostheses have been developed for patients requiring valve replacement.1,2
Mitral valvotomy for mitral stenosis predated the introduction of heart valve replacement, and valvotomy can now usually be achieved percutaneously with balloon dilatation in selected cases. Additionally techniques for repair of the diseased mitral valve, particularly mitral valve prolapse, have been developed and refined, avoiding the need for valve replacement. The morbidity, mortality, and long term results of valvotomy or valve repair in suitable patients are better than for valve replacement and should be used in preference when possible. Choice of operation and the prosthesis used for those undergoing valve replacement is important for each individual patient and ideally should be made together by the patient, cardiologist, and surgeon. This article deals with choice of prosthesis for the individual patient. Other articles in this series deal with the medical management of valvar heart disease,3 anticoagulant control,4 late results and late complications of valve replacement,5 and management of endocarditis.6
TYPES OF PROSTHESIS AVAILABLE
Mechanical prostheses
Ball valves
The original Starr-Edwards prosthesis comprised a silastic ball which seated in the sewing ring when closed and moved forward into the cage when open (fig 1). The original design has gone through several modifications but the basic design remains similar to the original. More than 200 000 have been implanted.
Figure 1.
Common types of heart valve prostheses: St Jude's Medical bileaflet (top left); Starr-Edwards ball and cage (top right); Bjork-Shiley tilting disc (bottom right); stented porcine prosthesis (bottom left).
Disc valves
The Bjork-Shiley prosthesis is comprised of a single graphite disc coated with pyrolite carbon which tilts between two struts of the housing which is made of stainless steel or titanium (fig 1). The original design was modified in the early 1980s to increase the angle of opening and to change the disc to a convexo-concave shape (cc model). This design change in conjunction with changes in the manufacturing process led to some models of this generation of the prostheses being prone to fracture of one of the retaining struts, allowing the disc to escape with catastrophic results. Although these structural defects were corrected and modified versions of the valve were subsequently implanted for several years, the Bjork-Shiley valve is no longer manufactured. More than 360 000 Bjork-Shiley prostheses have been implanted. Other manufacturers continue to produce single disc prostheses—for example, the Medtronic-Hall and the Aortech Ultracor.
Bileaflet valves
Bileaflet valves have two semicircular leaflets which open and close creating one central and two peripheral orifices. The St Jude medical valve (fig 1) was introduced in 1977, and more than 600 000 have been implanted. It and similar valves produced by other manufacturers are now the most commonly implanted type of mechanical prosthesis in the world.
Biological prostheses
All mechanical prostheses have an absolute requirement for anticoagulant treatment. The potential advantage of avoiding the hazards of anticoagulation has led to the search for a valve replacement of suitable biological material which would not require long term anticoagulant treatment. A number of different approaches to the problem of finding a suitable biological valve have been made. An autologous or autogeneous valve is fashioned from the patient's own tissue such as fascia lata or pericardium. An autograft valve is one translocated from one position to another—for example, when the patient's own pulmonary valve is used to replace a diseased aortic valve. A homograft (or allograft) valve is one transplanted from a human donor. A heterograft (or xenograft) valve is one transplanted from another species such as a pig, or manufactured from tissue such as bovine pericardium.
Autologous valves
In the 1970s valves were fashioned freehand from the patient's own fascia lata in the operating theatre. The procedure was technically demanding, the valves had very limited durability, and this approach has been abandoned. More recently frame mounted valves constructed from the patient's pericardium in the operating room using a commercially produced kit have been developed—for example, the Carpentier-Edwards Perimount pericardial prosthesis.
Autograft valves
Described by Donald Ross in 1967 the Ross procedure involves replacing the patient's diseased aortic valve with their own pulmonary valve which is in turn replaced by a homograft. The procedure is of particular value in children as the translocated pulmonary trunk grows with the child. Most late problems have been related to failure of the pulmonary homograft. The procedure requires a double valve replacement at operation with attendant increased surgical risk.
Homograft valves
Homografts from human cadavers (also known as allografts) have been used in some centres for aortic valve replacement for over 30 years. They are sterilised using an antibiotic solution and either stored in fixative or cryopreserved. Viable homografts are also successfully harvested from brain dead organ donors or from the explanted heart of a heart transplant patient.
Porcine heterograft (or xenograft) valves
Porcine valves are treated with glutaraldehyde which both sterilises the valve tissue and renders it biologically acceptable to the recipient—for example, the Hancock II Porcine (Medtronic) and the Biocor Porcine (St Jude Medical) prostheses. Most bioprosthesis are mounted on stents attached to a sewing ring (fig 1), but more recently stentless valves which are sewn in free hand have become available. Stentless valves have a greater effective orifice area compared with stented valves, but are technically more difficult to implant.
Bovine pericardial valves
These valves are fashioned from bovine pericardium mounted on a stented frame. The Ionescue-Shiley pericardial valve proved less durable than porcine valves and has been withdrawn. The Carpentier-Edwards pericardial valve is fabricated by anchoring the pericardial tissue behind the stents rather than using stitches through the tissue, as proved to be a weakness in the Ionescue-Shiley valve, but long term durability remains to be proven.
STUDIES EVALUATING DIFFERENT TYPES OF MECHANICAL PROSTHESES
Most studies of results of mechanical valve replacement have been observational studies of the results of valve replacement with one type of prosthesis. Most have shown excellent long term results for prosthesis survival, with no difference in durability between types of prosthesis. There have been few randomised controlled trials comparing outcomes after mechanical valve replacement. Thromboembolism has been reported as occurring at a higher rate following Starr-Edwards replacement than Bjork-Shiley. Bileaflet prostheses such as the St Jude valve appear to have the lowest risk of thromboembolism. Rates of thromboembolism are higher following mitral valve replacement than following aortic valve replacement.1,2
STUDIES EVALUATING DIFFERENT TYPES OF BIOLOGICAL PROSTHESES
As with mechanical valve prostheses, most studies have been observational, reporting results with one type of prosthesis. Several studies have identified porcine valve failure seven or more years after implantation, particularly in younger patients.1,2,7 One study compared results with stentless porcine prostheses with stented prostheses in the aortic position in a non-randomised case–controlled study of patients undergoing aortic valve replacement, and showed apparently enhanced durability of the stentless prosthesis.8 Advocates of the stentless prosthesis point to its superior haemodynamics with an effective valve area some 10% larger than a stented prosthesis of equivalent size. How relevant this is in clinical practice when the vast majority of patients undergoing aortic valve replacement for calcific aortic stenosis are in their 60s, 70s or 80s is doubtful. To answer properly the question of whether stentless prostheses give superior long term results, a randomised controlled trial is needed.
STUDIES COMPARING MECHANICAL WITH BIOLOGICAL PROSTHESES
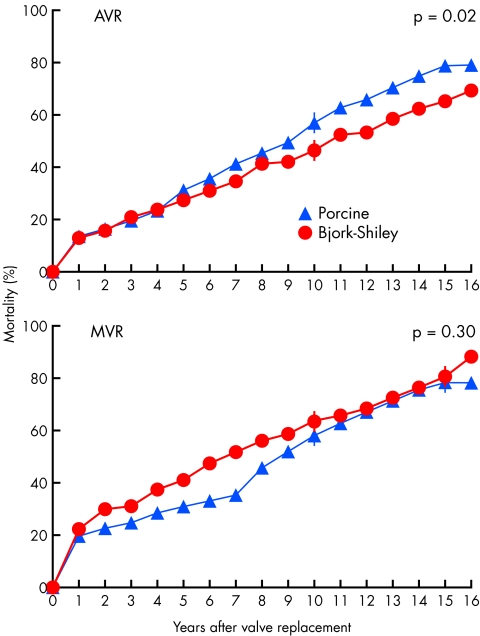
There have been two large randomised trials comparing results of mechanical valve with porcine valve replacement. Both trials used the Bjork-Shiley mechanical valve before the introduction of the convexo-concave model which subsequently proved liable to strut fracture. The Department of Veterans Affairs (VA) trial randomised 575 male patients undergoing single valve replacement in 13 centres between 1977 and 1982 to receive either a Bjork-Shiley tilting disc prosthesis or a Hancock porcine prosthesis. Three hundred and ninety four patients underwent aortic valve replacement and 181 patients mitral valve replacement. All patients receiving the Bjork-Shiley prosthesis received anticoagulants, but for those patients receiving a porcine prosthesis only those requiring anticoagulants for another reason (for example, atrial fibrillation) received warfarin. After a mean duration of follow up of 15 years Hammermeister and colleagues9 reported significantly improved survival at 15 years for those who had undergone aortic valve replacement with a Bjork-Shiley prosthesis (79% v 66%), but no significant difference for those who had undergone mitral valve replacement (fig 2). There was a significantly increased risk of reoperation with the Hancock prosthesis, both for patients who had undergone aortic valve and mitral valve replacement. There was no significant difference in the occurrence of thromboembolism or endocarditis, but there was a significantly greater occurrence of major bleeding with those receiving a Bjork-Shiley prosthesis as a result of the greater use of anticoagulants.
Figure 2.
The Veterans Affairs randomised trial comparing outcome following valve replacement with a mechanical (Bjork-Shiley) versus a porcine bioprosthetic valve. Cumulative mortality curves show significantly higher mortality over 15 years of follow up with bioprostheses for those undergoing aortic valve replacement (AVR). For mitral valve replacement (MVR) cumulative mortality was initially higher with mechanical prostheses but with more prolonged follow up the mortality curves converged. Reproduced from Hammermeister K, et al. J Am Coll Cardiol 2000:36;1152–8, with permission of the publisher.
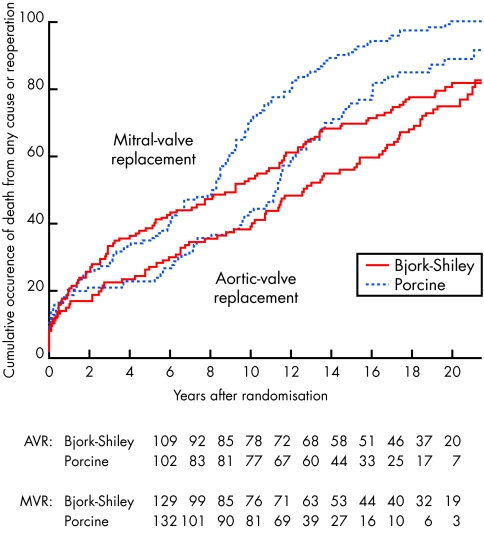
The Edinburgh heart valve trial randomised male and female patients undergoing valve replacement between 1975 and 1979 to receive a Bjork-Shiley or porcine (Hancock or Carpentier-Edwards) prosthesis.10 After a mean follow up period of 20 years, we reported results in 533 patients; 261 patients who had undergone mitral valve replacement, 211 aortic replacement, and 61 combined aortic and mitral valve replacement.11 We found no difference in patient survival between biological or mechanical valve recipients when all patients were considered together or when the subgroups undergoing aortic valve replacement, mitral valve replacement, and combined aortic and mitral valve replacement were considered separately. As in the VA study we found an increased need for reoperation with the porcine prostheses. An actuarial analysis using death or reoperation as combined end points showed a lower event rate and therefore improved valve survival with the Bjork-Shiley prosthesis. The increased need for re-operation for valve failure occurred after 8–10 years in those who had received a porcine mitral prosthesis, and at 10–12 years in those who had received a porcine aortic prosthesis (fig 3). Interestingly, when a patient who had received combined aortic and mitral valve replacement with porcine prosthesis required reoperation for valve failure, it was invariably the mitral prosthesis which had failed. There was a significantly increased risk of bleeding in those with the Bjork-Shiley prosthesis. When we performed an analysis examining the combined end points of death, reoperation, endocarditis, major embolism, and major bleeding as end points, we found survival free from major events was significantly better in those who received a Bjork-Shiley prosthesis.
Figure 3.
The Edinburgh heart valve trial. Cumulative occurrence of death or reoperation for patients undergoing aortic valve replacement or mitral valve replacement. Significantly more patients receiving a porcine prosthesis had one of these events compared with those receiving the mechanical Bjork-Shiley prosthesis. The curves separated at 8–10 years for mitral valve replacement and at 10–12 years for aortic valve replacement.
INFLUENCE OF PATIENT'S AGE ON DURABILITY OF PORCINE PROSTHESES
Biological valves have a higher failure rate in younger patients. Burdon and colleagues7 found that after 15 years of follow up only a third of patients who had received a bioprosthesis for aortic valve replacement between the ages of 16–39 years remained free of structural valve deterioration, compared with more than 90% of those over 70 at the time of implantation. In the Edinburgh trial we found an increased risk of porcine valve failure in younger patients with a relative risk of approximately 1.5 for every 10 years of age. Bioprostheses for mitral valve replacement have proved less durable than for aortic valve replacement in all age groups.
Peterseim and colleagues12 reported a large non-randomised series from a single centre of predominantly elderly patients undergoing aortic valve replacement with the patients receiving either a mechanical or porcine prosthesis. There was no difference in prosthesis survival between mechanical and porcine prostheses up to 10 years after implantation. Beyond 10 years an increased need for reoperation became apparent in the patients who had received a porcine prosthesis. The risk of bleeding was significantly increased in those who had received a mechanical prosthesis. However, at 10 years patient survival was only 50%, and in this older population with relatively limited life expectancy and low incidence of porcine prosthetic valve failure, aortic valve replacement with a porcine prosthesis appeared to confer an advantage compared with mechanical prostheses.
RISKS OF ANTICOAGULATION
The risks of anticoagulation are influenced by the intensity of treatment, the propensity for bleeding in the population studied, and the accuracy in identifying complications of anticoagulant treatment within the population studied. Before the introduction of the international normalised ratio (INR) intensity of anticoagulant treatment was not directly comparable between countries, or even within the same country. In the USA during the 1970s and 1980s higher average doses of warfarin were prescribed compared with the UK and other European countries. This followed a switch from human brain to rabbit brain thromboplastin in commercially available kits for measuring prothrombin time. Complication rates for anticoagulant treatment from this era in the USA were often higher than those reported from the UK and Europe, and higher than present studies in the USA.13
In the large trials of anticoagulation for non-rheumatic atrial fibrillation, in which there was optimal surveillance of anticoagulant control, much lower complication rates for anticoagulation were reported. Of these trials, those involving a high proportion of very elderly patients and higher intensities of anticoagulation reported higher complication rates. Modern bileaflet mechanical prostheses have been shown to function safely at low levels of anticoagulation similar to those used in most of the atrial fibrillation trials. One randomised trial compared high and low intensities of anticoagulation in patients undergoing aortic valve replacement with the St Jude or Omnicarbon bileaflet mechanical prosthesis. All patients included in this trial were at low risk of thromboembolism as they were in sinus rhythm and had a small left atrium on echocardiography. The trial showed that a target INR of 2.0–3.0 was as effective as a target INR of 3.0–4.5 in preventing valve thrombosis and thromboembolism, but at a significantly lower risk of bleeding.14 However, in unselected patients undergoing aortic valve replacement with the St Jude bileaflet mechanical prosthesis, thromboembolic events were only seen in patients with an INR of <2.5, and a target INR of 2.5–3.0 is therefore normally recommended for patients with bileaflet mechanical valves in the aortic position.15 Patients undergoing mitral valve replacement are at a higher risk of thromboembolism than those undergoing aortic valve replacement; left atrial size is frequently increased and atrial fibrillation is common. A higher target INR of 3.0–3.5 is therefore recommended for patients with bileaflet mechanical valves in the mitral position.15 A target of 3.0–4.5 is recommended for Starr-Edwards ball and cage and Bjork–Shiley single disc protheses.15
The addition of low dose aspirin (75–100 mg daily) at these levels of anticoagulation appears to confer additional protection against thromboembolism, with only a small increased risk of bleeding. A recent meta-analysis of randomised trials comparing aspirin and warfarin with warfarin alone showed significant benefit with the combination of treatment.16 Aspirin may be of particular advantage in patients who have undergone concomitant coronary bypass surgery at the time of valve replacement.
CHOICE OF VALVE PROSTHESIS FOR THE INDIVIDUAL PATIENT
In synthesising the results from these trials and observational studies, how should we advise individual patients on what type of prosthesis is most suitable for them? For most patients undergoing mitral valve replacement who are in atrial fibrillation and already on anticoagulant treatment the advice is easy. Bioprosthetic valves confer no advantage as the patient will continue anticoagulant treatment. Mechanical prostheses have better durability: modern bileaflet valves have good long term durability and can safely be managed with low intensity warfarin, and appear to be the optimal choice. Even for the minority of patients requiring mitral valve replacement who remain in sinus rhythm unless elderly or at risk from anticoagulant treatment, the enhanced durability of mechanical prostheses and the likelihood of atrial fibrillation developing with the passage of time would make a mechanical prosthesis the better choice. In replacing the valve the surgeon should try to conserve the subvalvar apparatus as this helps to preserve left ventricular function and appears to improve long term results.
For patients undergoing aortic valve replacement, choice of prosthesis is easier for the elderly patient. Bioprosthetic valves degenerate more slowly in elderly patients than in the young and the risks of anticoagulation may be higher in the very elderly. If an elderly patient would not be expected to live for more than 10 years following aortic valve replacement then a bioprosthesis would be the best choice, as the patient would avoid the risks of anticoagulant treatment. This strategy carries the risk that some patients will outlive their prosthesis and face the need for repeat surgery in their 80s with increased mortality and morbidity following reoperation at this age. It is difficult to choose an arbitrary age at which this strategy could be adopted; the American Heart Association/American College of Cardiology (AHA/ACC) task force recommends bioprostheses for those over 65 undergoing aortic valve replacement.
For younger patients undergoing aortic valve replacement a modern bileaflet mechanical valve would seem the optimal choice. Those wishing to, or needing to, avoid anticoagulant treatment could have a bioprosthesis. Aortic homografts may be more durable than porcine bioprostheses, particularly in younger patients, and seem to produce the best results with a short harvest and implantation time when the homograft is obtained from a brain dead organ donor or a heart transplant recipient. In one large series involving 618 patients, freedom from reoperation for valve failure at 10 years was 81% and at 20 years was only 35%. Homografts from donors older than 65 years of age, or where the donor was more than 10 years older than the recipient, had poorer results. It was also found that using the homograft to replace the valve and the aortic root with reimplantation of the coronary arteries produced better long term results than using the homograft for a subcoronary valve replacement.17 Repeat surgery for valve failure in patients with reimplanted coronaries is, however, much more demanding. Patients with renal failure, or with hypercalcaemia, have accelerated degeneration of bioprosthesis and should not receive a bioprosthesis. The AHA/ACC task force recommendations are shown in table 1. Additionally, some authorities advocate the use of homografts for patients with active endocarditis requiring valve replacement.
Table 1.
Summary of class I and II AHA/ACC recommendations for choice of prosthetic valve
| Recommendations for valve replacement with a mechanical prosthesis | Class |
| 1. Patients with expected long life spans | I |
| 2. Patients with a mechanical prosthetic valve already in place in a different position than the valve to be replaced | I |
| 3. Patients in renal failure, on haemodialysis, or with hypercalcaemia | II |
| 4. Patients requiring warfarin treatment because of risk factors* for thromboembolism | IIa |
| 5. Patients ≤65 years for AVR and ≤70 years for MVR | IIa |
| Recommendations for valve replacement with a bioprosthesis | |
| Class I There is evidence and/or general agreement that a given procedure or treatment is useful and effective. | |
| Class II There is conflicting evidence and/or a disagreement of opinion about the usefulness/efficacy of a procedure or treatment. | |
| Class IIa Weight of evidence/opinion is in favour of usefulness/efficacy. | |
| Class IIb Usefulness/efficacy is less well established by evidence/opinion. | |
| *Risk factors: atrial fibrillation, severe left ventricular dysfunction, previous thromboembolism, and hypercoagulable condition. | |
| AVR, aortic valve replacement; MVR, mitral valve replacement. | |
| 1. Patients who cannot or will not take warfarin treatment | I |
| 2. Patients ≥65 years needing AVR who do not have risk factors for thromboembolism* | I |
| 3. Patients considered to have possible compliance problems with warfarin treatment | IIa |
| 4. Patients >70 years needing MVR who do not have risk factors for thromboembolism* | IIa |
| 5. Valve rereplacement for thrombosed mechanical valve | IIb |
WOMEN OF CHILDBEARING AGE
For young women of childbearing age, wherever possible severe valvar lesions likely to cause problems during pregnancy should be corrected before pregnancy by treatments which avoid valve replacement—balloon valvuloplasty for mitral stenosis, mitral valve repair for mitral valve prolapse. If valve replacement is required the choice of type of prosthetic valve is difficult. Implantation of a bioprosthetic valve in the mitral position will confer the near certainty that the valve will degenerate in the patient's lifetime and require replacement, and the patient will face significant risk of mortality and morbidity at reoperation. This is likely to occur when the patient's children are still young. Pregnancy may accelerate the rate of bioprosthetic valve degeneration.
Implantation of a mechanical valve will necessitate warfarin treatment with an attendant risk of fetal loss or malformation and a maternal risk of valve thrombosis and peripartum haemorrhage. Warfarin crosses the placenta and is associated with an increased incidence of spontaneous abortion, stillbirth, prematurity, and embryopathy. The risk of warfarin embryopathy has been estimated at between 4–10% and appears to be dose dependent.18 In a recent observational study from Italy, Vitale and colleagues reported an overall risk of fetal complications as being four times higher in women requiring an average daily dose of > 5 mg warfarin compared with those requiring 5 mg daily.19 These authors therefore recommended for patients requiring low doses of warfarin a strategy of maintaining warfarin throughout pregnancy and an elective caesarean at 38 weeks.
Heparin does not cross the placental barrier and for this reason has been considered to be safer for the fetus. However, the risk of thromboembolic complications including fatal valve thrombosis in patients treated with subcutaneous heparin has been observed in some studies to be between 12–24%. The European Society of Cardiology guidelines for prevention of thomboembolic events in valvar heart disease therefore recommend that women at high risk because of a history of previous thromboembolism or an older generation prosthesis in the mitral position who chose not to take warfarin during the first trimester should receive continuous unfractionated heparin intravenously throughout the first trimester (table 2).20 Low molecular weight heparin has been safely used for deep vein thrombosis in pregnancy, is obviously far more convenient than continuous unfractionated heparin, but has yet to be fully evaluated during pregnancy in patients with mechanical prosthetic valves.
Table 2.
Recommendations20 for anticogulation during pregnancy in patients with mechanical prosthetic valves: weeks 1–35
| Indication | Class |
| 1. The decision whether to use heparin during the first trimester or to continue oral anticoagulation throughout pregnancy should be made after full discussion with the patient and her partner; if she chooses to change to heparin for the first trimester, she should be made aware that heparin is less safe for her, with a higher risk of both thrombosis and bleeding, and that any risk to the mother also jeopardises the baby* | I |
| 2. High risk women (a history of thromboembolism or an older generation mechanical prosthesis in the mitral position) who choose not to take warfarin during the first trimester should receive continuous unfractionated heparin intravenously in a dose to prolong the midinterval (6 hours after dosing) aPTT to 2–3 times control. Transition to warfarin can occur thereafter | I |
| 3. In patients receiving warfarin, INR should be maintained between 2.0–3.0 with the lowest possible dose of warfarin, and low dose aspirin should be added | IIa |
| 4. Women at low risk (no history of thromboembolism, newer low profile prosthesis) may be managed with adjusted dose subcutaneous heparin (17 500–20 000 U twice daily) to prolong the mid interval (6 hours after dosing) aPTT to 2–3 times control. | IIb |
Class I There is evidence and/or general agreement that a given procedure or treatment is useful and effective.
Class IIa Weight of evidence/opinion is in favour of usefulness/efficacy.
Class IIb Usefulness/efficacy is less well established by evidence/opinion.
From the European Society of Cardiology guidelines for prevention of thromboembolic events in valvular heart disease.20
aPPT, activated partial prothrombin time; INR, international normalised ratio.
The risks of anticoagulation with warfarin are increased in patients in whom compliance is poor. North and colleagues reviewed outcomes in 232 females aged 12–35 years who underwent valve replacement between 1972 and 1992 in Auckland, New Zealand.21 The 10 year survival of patients with mechanical (n = 178), bioprosthetic (n = 73), and homograft (n = 72) valves was 70%, 84%, and 96%, respectively, with wide confidence intervals. After adjusting for other variables the relative risk of death with mechanical compared with bioprosthetic valves was approximately 2. Thromboembolism occurred significantly more commonly in patients with mechanical prostheses, with 45% having had a thromboembolic event by five years compared with 13% for bioprosthetic valves. The authors noted that there was a high proportion of patients in this study in whom compliance with medication was not ideal. Reoperation for valve failure occurred significantly more often in patients with bioprostheses than with mechanical or homograft prostheses. There were 132 pregnancies in 71 of the patients, and although no details of the outcome of the pregnancy were given, in this study pregnancy appeared not to increase structural deterioration or reduce survival of bioprosthetic valves.
The best management strategy for women of childbearing age requiring valve replacement remains unclear. Both they and their spouses must be fully informed of the risks of each strategy before undergoing valve replacement surgery.
Acknowledgments
I am grateful to Ole Lund for his constructive comments.
REFERENCES
- 1.Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med 1996;335:407–16. ▸ A useful and up to date review of different types of prosthetic valve presently in use. This review covers the auscultatory characteristics of different types of prosthesis, and radiological and echocardiographic assessment. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello B, DeLeon AC, et al. ACC/AHA practice guidelines. Guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol 1998;32:1486–588. ▸ A comprehensive review of all aspects of the management of patients with valvar heart disease including valve replacement surgery with more than 700 references. Available in full text from www.acc.org.9809971 [Google Scholar]
- 3.Boon NA, Bloomfield P. Medical management of valvar heart disease. Heart 2002;87:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohlke-Barwolf C. Anticoagulation in valvar heart disease: new aspects and management during non-cardiac surgery. Heart 2000;84:567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groves P. Surgery of valve disease: late results and late complications. Heart 2001;86:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakley CN, Hall RH. Endocarditis: problems—patients being treated for endocarditis and not doing well. Heart 2001;85:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdon TA, Miller DC, Oyer PE, et al. Durability of porcine valves fifteen years in a representative North American patient population. J Thorac Cardiovasc Surg 1992;103:238–51. ▸ A report on a large series of patients which identified an increased failure rate of porcine prostheses in younger patients. [PubMed] [Google Scholar]
- 8.David TE, Puschmann R, Ivanov J, et al. Aortic valve replacement with stentless and stented porcine valves: a case-match study. J Thorac Cardiovasc Surg 1998;116:236–41. ▸ A non-randomised study showing a trend to superior results with stentless prostheses. [DOI] [PubMed] [Google Scholar]
- 9.Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152–8. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield P, Wheatley DJ, Prescott RJ, et al. Twelve-year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. N Engl J Med 1991;324:573–9. [DOI] [PubMed] [Google Scholar]
- 11.Oxenham H, Bloomfield P, Wheatley DJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart (in press). ▸ These two randomised controlled trials confirmed the increased risk of valve failure with biological valves compared with mechanical valves although with an increased risk of bleeding with mechanical valves. The VA trial demonstrated significantly better survival in those undergoing mechanical aortic valve replacement.
- 12.Peterseim D, Can Y-Y, Cheruvu S, et al. Longterm outcome after biological versus mechanical aortic valve replacement in 841 patients. J Thorac Cardiovasc Surg 1999;117:890–7. ▸ A large non-randomised series demonstrating similar long term (10 years) results with porcine prostheses compared with mechanical prostheses in the aortic position in patients over 65 years of age. [DOI] [PubMed] [Google Scholar]
- 13.Rosendaal FR. The scylla and charybdis of oral anticoagulant treatment. N Engl J Med 1996;335:587–9. ▸ A succinct review of the use of warfarin treatment at different levels of intensity of anticoagulation and the relative risks of haemorrhage and thromboembolism. [DOI] [PubMed] [Google Scholar]
- 14.Acar J, Iung B, Boissel JP, Samama MM, et al. AREVA: multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation 1996:94:2107–12. ▸ This study showed that in patients at low risk of embolism (aortic valve replacement in sinus rhythm), for bileaflet mechanical valves a target INR of 2.0–3.0 was as effective as a target INR of 3.0–4.5 with a significantly lower risk of bleeding. [DOI] [PubMed] [Google Scholar]
- 15.British Cardiac Society and Royal College of Physicians. Valvular heart disease; investigation and management. Recommendation of a working group of the British Cardiac Society and the research unit of the Royal College of Physicians. London: Royal College of Physicians, July 1996. ▸ A useful guideline for the management of patients with valvar heart disease. [PMC free article] [PubMed]
- 16.Masssel D, Little SH. Risks and benefits of adding anti-platelet therapy to warfarin among patients with prosthetic heart valves: a meta-analysis. J Am Coll Cardiol 2001;37:569–78. ▸ A useful overview and meta-analysis of the use of warfarin and aspirin in patients with prosthetic valves. It concludes that combining low dose aspirin with warfarin decreases the risk of systemic embolism or death in patients with prosthetic heart valves, with only a slightly increased risk of bleeding. [DOI] [PubMed] [Google Scholar]
- 17.Lund O, Chandrasekaran R, Grocott-Mason H, et al. Primary aortic valve replacement with allografts over twenty-five years: valve-related and procedure-related determinants of outcome. J Thorac Cardiovasc Surg 1999;1117:71–91. ▸ One of the largest series of patients undergoing valve replacement surgery using allografts (homografts) with a very long period of follow up of the patients receiving these valves. [DOI] [PubMed] [Google Scholar]
- 18.Hanania G. Management of anticoagulants during pregnancy. Heart 2001;86:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale N, De Feo M, De Santo LS, et al. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol 1999;33:1637–41. [DOI] [PubMed] [Google Scholar]
- 20.Gohlke-Barwolf C, Acar J, Oakley C, et al. Guidelines for prevention of thromboembolic events in valvular heart disease: study group of the working group on valvular heart disease of the European Society of Cardiology. Eur Heart J 1995;16:1320–30. [DOI] [PubMed] [Google Scholar]
- 21.North RA, Sadler L, Stewart AW, et al. Long-term survival and valve-related complications in young women with cardiac valve replacements. Circulation 1999;99:2669–76. ▸ An observational study of outcome of valve replacement surgery in 255 young women, 71 of whom became pregnant and had children during the period of study. [DOI] [PubMed] [Google Scholar]