Because of its high success rate and low morbidity, radiofrequency (RF) catheter ablation has become first line treatment for many arrhythmias. In this procedure, one or more electrode catheters are advanced percutaneously through the vasculature to contact cardiac tissues. A diagnostic study is performed to define the arrhythmia mechanism, and subsequently an ablation catheter is positioned adjacent to the arrhythmogenic substrate. Radiofrequency energy of up to 50 W is delivered in the form of a continuous unmodulated sinusoidal waveform, typically for 60 seconds. Energy delivery is well tolerated by a mildly sedated patient, and results in a small (5 mm) well circumscribed lesion. Destruction of tissue critical for arrhythmogenesis (such as an accessory pathway) and its subsequent replacement with scar eliminates arrhythmia.
The small size of radiofrequency lesions has led to the greatest success in the treatment of those arrhythmias that have a focal origin or depend on a narrow isthmus for maintenance. Furthermore, since precise lesion placement is required, arrhythmias for which ablation is most effective (accessory pathways, atrioventricular nodal re-entry tachycardia (AVNRT)) have largely anatomically based or directed substrates. Accessory pathways are anomalous epicardial connections between the atria and ventricles, and are located along the mitral or tricuspid valve annulus, reducing the problem of localisation to identification of a point on a line. An electrode catheter in the coronary sinus outlines the mitral annulus fluoroscopically, and is used to guide ablation catheter position. The relative amplitude of the atrial and ventricular components of the bipolar electrogram recorded by the ablation catheter further defines tip position relative to the annulus. Earliest atrial or ventricular activation during pathway conduction identifies pathway location along the annulus. The target for catheter ablation of AVNRT (the AV nodal slow pathway) occurs even more predictably in the posteroseptum. Ablation may be guided entirely by anatomic location relative to His and coronary sinus catheter positions, which serve as fluoroscopic landmarks, or by a combined anatomic and electrogram approach. Detailed discussions of radiofrequency ablation are available elsewhere.1
ROLE OF MAPPING SYSTEMS
The high success of catheter ablation in the treatment of AVNRT and accessory pathways, and atrioventricular junction ablation for rate control in atrial fibrillation, has led to interest in application of this therapy to a broad array of arrhythmias. Success in stable arrhythmias with predictable anatomic locations or characteristics identifying endocardial electrograms, such as idiopathic ventricular tachycardia or isthmus dependent atrial flutter, has approached 90%. However, ablation of more complex arrhythmias, including some atrial tachycardias, many forms of intra-atrial re-entry, most ventricular tachycardias, and atrial fibrillation continues to pose a major challenge. This stems in part from the limitations of fluoroscopy and conventional catheter based mapping techniques to localise arrhythmogenic substrates that are removed from fluoroscopic landmarks and lack characteristic electrogram patterns. The inability to associate accurately the intracardiac electrogram with a specific endocardial site also limits the reliability with which the roving catheter tip can be placed at a site that was previously mapped. This results in limitations when the creation of long linear lesions is required to modify the substrate, and when multiple isthmuses or “channels” are present. Additionally, since in conventional endocardial mapping a single localisation is made over several cardiac cycles, the influence of beat-to-beat variability on overall cardiac activation cannot be known. Transient or haemodynamically unstable arrhythmias are also not mappable by conventional techniques. With prolonged procedures, there is increased exposure to ionising radiation, adding risk for both the patient and physician.
New techniques in catheter localisation and arrhythmia mapping have been developed to overcome these limitations and expand the list of arrhythmias amenable to catheter ablation (table 1). These include multi-electrode baskets, electroanatomical mapping, and non-contact mapping (table 2). The mechanism of operation and clinical experience with these mapping tools will be reviewed.
Table 1.
Role of advanced mapping systems based on arrhythmia
| Limited role for advanced mapping (high conventional success rate) | Advanced mapping shortens procedure, limits fluoroscopy, or enhances success | Advanced mapping extremely helpful or essential |
| AVNRT | Typical atrial flutter | Macroreentrant atrial arrhythmias after surgical correction of congenital heart disease |
| Accessory pathway ablation | Idiopathic ventricular tachycardia (RVOT, LVOT, fascicular VT) | Transient/multiple focal atrial tachycardias |
| AV junction ablation (for rate control in atrial fibrillation) | Repeat ablation after previously failed attempt | Haemodynamically unstable VT |
| Haemodynamically stable VT (non-idiopathic) | Atrial fibrillation: linear lesions for atrial compartmentalisation procedures; also useful, but role less defined for encircling pulmonary vein isolation and non-pulmonary vein focus localisation |
AVNRT, atriventricular nodal re-entrant tachycardia; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract, VT, ventricular tachycardia.
Table 2.
Mapping system characteristics
| Multi-electrode baskets | Electroanatomical mapping | Non-contact mapping | |
| Parallel data acquisition (shorten procedure time) | Yes | No | Yes |
| Map resolution | Limited (1 cm) | Medium to High* | High |
| Non-fluoroscopic catheter navigation | No | Yes | Yes |
| Transient arrhythmia mapping | Yes | No | Yes |
| Substrate (bipolar voltage) mapping | No | Yes | No |
| Catalogue ablation points (guide linear lesion creation) | No | Yes | Yes |
| Find gap in linear lesion | No | Yes† | Yes |
*Function of time spent/number of points collected.
†Time consuming—line must be retraced with mapping catheter.
MULTI-ELECTRODE (“BASKET”) CATHETER MAPPING
The mapping catheter consists of an open lumen catheter shaft with a collapsible, basket shaped, distal end. Currently basket catheters consist of eight equidistant metallic arms, providing a total of 64 unipolar or 32 bipolar electrodes capable of simultaneously recording electrograms from a cardiac chamber. The catheters are constructed of a superelastic material to allow passive deployment of the array catheter and optimise endocardial contact. The size of the basket catheter used depends on the dimensions of the chamber to be mapped, requiring antecedent evaluation (usually by echocardiogram) to ensure proper size selection. The collapsed catheters are introduced percutaneously into the appropriate chamber where they are expanded.
The mapping system consists of an acquisition module connected to a computer, which is capable of simultaneously processing: (1) 32 bipolar electrograms from the basket catheter; (2) 16 bipolar/unipolar electrograms signals; (3) a 12 lead ECG; and (4) a pressure signal. Colour coded activation maps are reconstructed on-line. The electrograms and activation maps are displayed on a computer monitor and the acquired signals can be stored on optical disk for off-line analysis. Activation marks are generated automatically with either a peak or slope (dV/dt) algorithm, and the activation times are then edited manually as needed.2
Clinical experience
Percutaneous endocardial mapping with multi-electrode basket shaped catheter has been shown to be feasible and safe in patients with ventricular tachycardia (VT) in coronary disease. Fragmented early endocardial activation—suggesting a zone of slow conduction that may be a suitable ablation target—is frequently demonstrated. However, the relatively large inter-electrode spacing in available catheters has prevented high resolution reconstruction of the re-entrant circuit in the majority of patients.3 More recently, a steerable sector basket catheter with improved spatial resolution (±1 cm) to guide ablation procedures in patients with postinfarction VT has been used. This has enabled demonstration of early endocardial activation and localisation of the area of slow conduction during VT.
Basket catheter strengths and limitations
The multi-electrode endocardial mapping system allows simultaneous recording of electrical activation from multiple sites and fast reconstruction of endocardial activation maps. This may limit the time endured in tachycardia compared to single point mapping techniques without the insertion of multiple electrodes and facilitate endocardial mapping of haemodynamically unstable tachycardias.
Because of its poor spatial resolution, the basket catheter in its current iteration has demonstrated only limited clinical utility to guide ablation of re-entrant atrial or ventricular arrhythmias. The spatial resolution (approximately 1 cm along the arms of the catheter and ≥ 1 cm between the arms) is generally not sufficient for a catheter based ablation procedure given the small size and precise localisation associated with radiofrequency lesions.3 The role of multi-electrode basket catheters in mapping smaller structures such as pulmonary veins for the treatment of focal atrial fibrillation (discussed below) may be more promising, but is not known.
ELECTROANATOMIC MAPPING
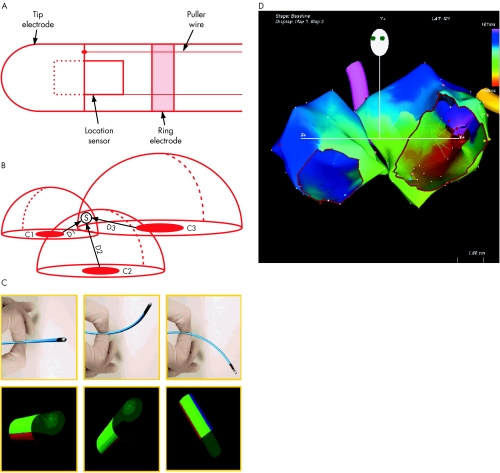
The CARTO system (Biosense, Diamond Bar, California, USA) correlates electrophysiologic characteristics with endocardial anatomy by continuously recording mapping catheter location. A locator pad placed beneath the operating table generates ultra-low intensity magnetic fields that code the mapping space around the patient's chest with spatial distinguishing characteristics. The pad's three coils each generate a magnetic field that decays in strength as a function of distance from that coil. A sensor embedded in the mapping catheter measures the strength of each magnetic field, enabling determination of the distance from each coil. These distances are the radii of theoretical spheres around each coil; the intersection of the three spheres defines the location of the sensor, and thus the catheter tip, in space (fig 1). In addition to catheter location, orientation (roll, pitch, and yaw) is determined.
Figure 1.
Electroanatomic mapping. (A) The catheter is composed of tip and ring electrodes and a location sensor embedded within the catheter. (B) A location pad with three coils (C1, C2, and C3) generates magnetic fields that decay as a function of distance from the coils. The sensor measures the strength of each field, permitting determination of the distance from each coil (D1, D2, D3). The intersection of three theoretical spheres of radii D1, D2, and D3 determines the catheter tip location in space. Reproduced from Gepstein et al,20 with permission. (C) Deflection of the catheter in space (top panels) results in real time display of catheter orientation on the computer screen, to guide non-fluoroscopic manipulation. (D) Activation map from a patient with left atrial figure of eight re-entrant tachycardia. The two atria are shown in the left anterior oblique view, with tricuspid valve and mitral valve cut out. The colour at each anatomic point shows local activation time relative to the reference catheter (scale top right).
With this system, a mapping catheter with tip and proximal electrodes to record unipolar and bipolar signals is advanced percutaneously to the chamber of interest. Catheter position is recorded relative to the location of a reference back patch, thus compensating for subject motion within the coils' fields. The mapping procedure involves positioning the mapping catheter at sequential points along the endocardium. Catheter tip location and electrograms are simultaneously acquiring while the catheter remains in stable contact with endocardium. Local activation times are calculated relative to the body surface ECG or a fixed (reference) intracardiac electrode. The system continuously monitors the quality of catheter–tissue contact and local activation time stability to ensure validity and reproducibility of each local measurement. The acquired information is then colour coded and displayed. As each new site is acquired, the reconstruction is updated in real time to progressively create a three dimensional chamber geometry colour encoded with activation time (fig 1). In addition to activation time maps, dynamic propagation maps displayed as movies of sequential activation on the computer workstation can be created. Additionally, the collected data can be displayed as voltage maps depicting the magnitude of the local peak voltage in a three dimensional model. These can be useful to define areas scarring and electrically diseased tissue. This system allows precise positioning of the catheter tip at a site of interest that was previously sampled, tagging of regions of interest, and marking positions of veins and valves.
Abbreviations.
AF: atrial fibrillation
AVNRT: atrioventricular nodal re-entrant tachycardia
MEA: multiple electrode array
RF: radiofrequency
VT: ventricular tachycardia
Clinical experience
Atrial tachycardia and flutter
Although catheter ablation of atrial tachycardia guided by standard radiographic imaging has provided effective treatment in some populations, mapping complexity may lead to prolonged procedure and fluoroscopy time. Conventional ablation has been even more challenging in patients with congenital heart disease and previous surgery, as macro-reentrant arrhythmias arise utilising critical channels of slow conduction present within scars, or between scars and anatomic boundaries. Approaches for ablation have included identification of isolated diastolic potentials and entrainment mapping to identify critical circuit components, and creation of linear lesions between atriotomy scar and an anatomic barrier (for example, tricuspid annulus or inferior vena cava) to interrupt the re-entrant circuit. The presence of multiple re-entrant circuits, electrically silent scar zones, fractionated and small potentials, and arrhythmogenic substrate location distant from fluoroscopic landmarks has limited long term procedural success.
Three dimensional non-fluoroscopic electroanatomical mapping has facilitated ablation of this arrhythmia. In patients with focal arrhythmia, detailed, high density mapping of the earliest endocardial activation site during tachycardia can be acquired—assuming the arrhythmia is sustained or frequently recurrent. The system has been highly successful in ablating ectopic atrial tachycardia rapidly and with a small number of RF applications.4 In the presence of structural heart disease, the system creates useful endocardial three dimensional maps with labelled structures (for example, valves, veins) to guide catheter manipulation. Local bipolar peak voltage maps have been successfully used to identify the complex substrate responsible for arrhythmia in the setting of congenital heart disease with prior corrective surgery. In these patients, multiple isolated channels between scars usually located in the right atrial free wall are responsible macro-reentrant atrial tachycardia. Focal ablation within channels guided by electroanatomical substrate maps eliminates arrhythmia.5
Typical human atrial flutter arises from a stable macro-entrant circuit produced utilising the sub-eustachian isthmus between the tricuspid valve annulus and the ostium of the inferior vena cava as its critical zone of slow conduction. Creation of a complete line of conduction block across the sub-eustachian isthmus eliminates counter clockwise (typical) and clockwise atrial flutter. Because of its well defined boundaries, sub-eustachian isthmus dependent flutter is usually easily treated with conventional techniques. Electroanatomical mapping has been used to confirm the anatomic location of the flutter circuit, to guide linear lesion creation, and to decrease fluoroscopy use.6 Additionally, electroanatomical mapping can identify gaps in the linear lesion in the setting of recurrent flutter after previous ablation, to guide repeat ablation.7 However, since the technique requires point to point data acquisition, the entire line must be retraced to locate the defect, which can be time consuming.
Atrial fibrillation
Curative non-surgical treatment of atrial fibrillation (AF) still remains a challenge. Currently, two approaches exist to treat AF: elimination or control of rapidly firing foci that trigger AF (mostly commonly from the pulmonary veins), or transcatheter creation of linear lesions to modify the substrate to prevent AF sustenance.8,9 Since the triggers that initiate AF are often only transiently active or rapidly lead to AF, point to point mapping as employed by the electroanatomical system is of limited utility for localising discharging foci. However, the navigation employed by the system may be useful for returning to sites at which pulmonary vein potentials are recorded. These potentials have been associated with muscle bundles that connect to or give rise to discharging foci; pulmonary vein potential by catheter ablation has resulted in elimination of arrhythmia in some patients. Experience with this use of the system is limited, and requires confirmation. When non-pulmonary vein foci are present, the utility of this system to localise them is quite limited.
Percutaneous linear lesion creation is based upon the surgical maze procedure and aims to compartmentalise the atria into sections too small to support wavefront re-entry. Initial attempts at emulating the surgical approach to treatment of AF in the catheter laboratory have met with limited success. While the three dimensional electroanatomical reconstruction of the targeted atrium and catheter navigation facilitate linear lesion creation by “tagging” ablation sites on the map, catheter “reach” remains challenging. Another technical challenge is assuring the “completeness” of ablation lines, since gaps that permit impulse conduction are often pro-arrhythmic and lead to incisional flutters. Electroanatomical mapping can confirm line integrity, but the entire length of the linear lesion must be retraced with the mapping catheter while pacing from a second site—a time consuming process. Efforts at transcatheter right atrial or biatrial compartmentalisation with this system have resulted in long procedures with highly variable success rates.9,10 More limited lesion sets to isolate circumferentially the pulmonary veins may hold greater promise.11
Ventricular tachycardia
Radiofrequency catheter ablation of VTs in the setting of previous myocardial infarction or other structural heart disease has been challenging because of the frequent presence of multiple re-entry circuits, haemodynamic instability during arrhythmia, frequent changes from one VT to another, and absence of reproducibly inducible VT. The small size of lesions created by RF ablation in the presence of large regions of abnormal substrate further limits treatment.
Electroanatomical activation maps, which must be acquired during tachycardia to define the circuit, are limited to stable arrhythmias amenable to acquisition of multiple sequential points. However, substrate (voltage) maps created during sinus rhythm have been used to define abnormal left ventricular endocardium in patients with drug refractory, monomorphic, unmappable VT and frequent implantable defibrillator shocks.12 Regions of “dense scar” are defined as those with a bipolar voltage amplitude < 0.5 mV. Placement of a median of four linear lesions with typical length 4 cm in a point by point manner from scar to anatomic boundaries or normal myocardium can effectively control arrhythmia in many patients with otherwise unmappable VT.12 Success with electroanatomical mapping is greater when at least one critical isthmus for tachycardia can be defined.13
Conventional approaches—activation sequence mapping, pace mapping or both—have had great success with ablation of focal VT, and advanced mapping is not usually required. However, electroanatomical mapping has been successfully used to guide ablation of patients with focal tachycardia in order to limit fluoroscopy. Additionally, electroanatomical mapping permits re-navigation of the mapping catheter to previous sites of ablation or mechanically terminated tachycardia locations to further facilitate ablation.
Electroanatomical mapping strengths and limitations
In the setting of stable or frequently repetitive arrhythmia, creation of high spatial resolution (< 1 mm) activation maps and the ability to localise the catheter tip relative to maps facilitates ablation of complex arrhythmias difficult to treat conventionally. Additionally, fluoroscopy time can be reduced via electromagnetic catheter navigation, and the catheter can be accurately guided to positions removed from fluoroscopic markers. In the absence of stable or high frequency arrhythmia, if arrhythmia arises in the setting of cardiac structural abnormalities, voltage maps have been very useful in defining the arrhythmogenic substrate. This technique has been used to guide ablation successfully in patients with re-entrant atrial and ventricular arrhythmias not amenable to conventional ablation treatment.
The sequential data acquisition required for map creation remains very time consuming, particularly if the integrity of long linear lesions is assessed, or if multiple stable arrhythmias require mapping. Since the acquired data are not coherent in time, multiple beats are required for creation of the activation map. Rapidly changing or transient arrhythmias (as seen in the triggers that give rise to focal atrial fibrillation) are not easily recorded, and can only be mapped if significant substrate abnormalities are present.
NON-CONTACT ENDOCARDIAL MAPPING
Non-contact mapping is based on the physical principle that when one three dimensional surface is placed within another, if the electrical potential on one surface is known, the potential on the other can be calculated. To map, a probe with known dimensions is advanced to the cardiac chamber of interest and, once there, expanded. The endocardial surface of the chamber of interest is defined at procedure, and the electrical potential present on the probe's surface recorded, permitting calculation of the endocardial potential. This allows reconstruction of electrograms at endocardial sites in the absence of physical electrode contact at those locations (virtual electrograms), enabling recording of cardiac electrical activity from thousands of points simultaneously.
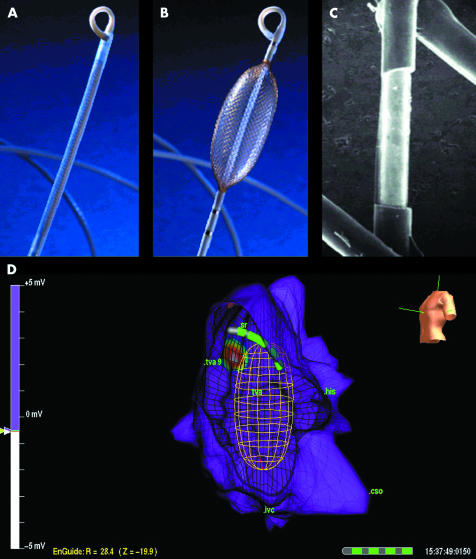
The non-contact mapping system (EnSite 3000, Endocardial Solutions, Inc, St Paul, Minnesota, USA) consists of catheter mounted multielectrode array (MEA) which serves as the probe, a custom designed amplifier system, and a computer workstation that is used to display three dimensional maps of cardiac electrical activity. The catheter consists of a 7.5 ml balloon mounted on a 9 French catheter around which is woven a braid of 64 insulated 0.003 mm diameter wires (fig 2). Each wire has a 0.025 mm break in insulation that serves as a non-contact unipolar electrode. The raw far-field electrocardiographic data from the MEA are acquired and fed into a multichannel recorder and amplifier system that also has 16 channels for conventional contact catheters, 12 channels for the surface ECG, as well as pressure channels. The unipolar MEA signals are recorded using a ring electrode as a reference, which is located on the shaft of the MEA catheter. An electrically based locator signal is also generated by the system to permit non-fluoroscopic navigation of any standard roving contact catheter used for ablation.
Figure 2.
Non-contact system multiple electrode array (MEA). (A) In low profile, the MEA is advance through the vasculature to the chamber of interest. (B) After deployment in the chamber of interest, the MEA is expanded to record intracavitary potentials. (C) Photomicrograph of the MEA showing one of the 64 laser etched laser etched unipolar electrodes. (D) Right atrial map in a patient with ectopic tachycardia. Left anterior oblique view is shown. Point of earliest activation is shown (white centre of target). On computer workstation, activation “movie” depicts wavefront propagation. The position of mapping catheter relative to the atrial geometry is shown by means of the locator signal.
The locator system locates any conventional catheter in space with respect to the MEA (and thus with respect to the cardiac chamber being mapped) by passing a 5.68 kHz, low current “locator” signal between the contact catheter electrode being located and reference electrodes on the non-contact array. This creates a potential gradient across the MEA electrodes used to position the source. This locator signal serves several purposes. Firstly, it is used to construct the three dimensional computer model of the endocardium (virtual endocardium) that is required for the reconstruction of endocardial electrograms and isopotential maps (fig 2). This model is acquired by moving a conventional, contact catheter around the cardiac chamber, building up a series of coordinates for the endocardium, and generating a patient specific, anatomically contoured model of its geometry. During geometry creation, only the most distant points visited by the roving catheter are recorded in order to ignore those detected when the catheter is not in contact with the endocardial wall. Geometric points are sampled at the beginning of the study during sinus rhythm, resulting in a contoured model with end diastolic dimensions. Secondly, the locator signal can be used to display and log the position of any catheter (for example, His catheter, coronary sinus catheter, and so on) on the endocardial model. Thirdly, during catheter ablation procedures, the locator system is used in real time to navigate the catheter to sites of interest identified from the isopotential colour maps, to catalogue the position of RF energy applications on the virtual endocardium, and to facilitate re-visitation of sites of interest by the ablation catheter.
The system reconstructs over 3360 electrograms simultaneously over a computer generated model of the chamber of interest (“virtual” endocardium). Because of the high density of data, colour coded isopotential maps are used to depict graphically regions which are depolarised, and wavefront propagation is displayed as a user controlled three dimensional “movie” (fig 2). Additionally, unipolar or bipolar virtual electrograms can be displayed by selection of an area of interest, and displayed as if from point, array, or plaque electrodes. The fidelity of virtual unipolar electrograms compared to actual contact electrograms has been confirmed in vitro and in vivo, as has the precision of the catheter navigation system.14
Clinical experience
Ectopic atrial tachycardia and atrial flutter
Non-contact mapping has been used to facilitate ectopic tachycardia ablation. As with the electroanatomical system, navigation to regions difficult to pinpoint fluoroscopically is facilitated, pertinent structures (such as the His bundle or valve annuli) can be annotated and localised in three dimensional space, and the ablation catheter can be accurately and repeatedly renavigated to predetermined sites in the cardiac chamber. Additionally, the high density parallel data acquisition permits mapping of arrhythmias seen only transiently in the electrophysiology laboratory, even in the absence of overt abnormalities in cardiac structure to guide substrate localisation. Once the geometry is defined, the origin of multiple arrhythmias can be rapidly determined. This is particularly useful for patients with multiple atrial tachycardia present.
As noted above, typical atrial flutter is usually readily treated using standard ablation techniques. However, non-contact mapping has been used to confirm the anatomic location of the flutter circuit, to reduce fluoroscopy time, and to confirm block in the setting of electrogram degradation subsequent to ablation.15 Non-contact mapping has also been used to identify and guide RF ablation of the site of residual conduction following incomplete linear lesion at the isthmus. Because of its ability to record from multiple sites simultaneously, the technique can rapidly identify gaps in linear lesions. This is accomplished from analysis of one or more paced complexes originating adjacent to the line being assessed. These global mapping capabilities have also facilitated ablation of atypical flutter. In a study of patients with congenital heart disease and previous Fontan procedure, non-contact mapping improved recognition of the anatomic and surgical substrate and identified exit sites from zones of slow conduction in all clinical arrhythmias.16
Atrial fibrillation
As described above, the two approaches used to treat AF include ablation of focal triggers and creation of linear lesions. Early clinical experience suggests non-contact mapping may play a role in both approaches.
Pulmonary vein foci have been identified as the triggers of paroxysmal and persistent AF.8 Because of the intermittent and transient nature of pulmonary vein discharges and rapid degeneration to atrial fibrillation, mapping for ablation has been difficult. Using its ability to globally map a single complex, non-contact mapping has been used to identify focal triggers and the bundles of myocardium connecting pulmonary vein to left atrial musculature.17 Additionally, since up to 30% of triggering foci may emanate from non-pulmonary vein foci (so that anatomical structure cannot readily guide ablation), non-contact mapping may be particularly useful in this setting, although its role is not established. Since focal discharges often occur during the T wave of the preceding ventricular complex, isopotential map interpretation may be challenging, as ventricular repolarisation potentials must be accounted for (or filtered out) in signal interpretation.
Non-contact mapping has also been used to guide linear lesion creation for the control of atrial fibrillation.18 The ability to confirm bidirectional block across the entire length of a long ablation line without the need to retrace the line has been very useful, and right atrial lesions have resulted in arrhythmia control with antiarrhythmic medications previously ineffective. However, as noted above, the role of transcatheter right atrial compartmentalisation remains uncertain, and left atrial compartmentalisation is limited in large measure by ablation energy delivery systems.
Ventricular tachycardia
As noted above, catheter ablation of re-entrant VT has been limited by the presence of multiple re-entry circuits, haemodynamic instability during arrhythmia, frequent changes from one VT to another, absence of reproducibly inducible VT, and the time required for sequential endocardial activation mapping.
Because of its ability to record cardiac activation from a single complex, non-contact mapping can facilitate the mapping of hemodynamically unstable rhythms. The multiple electrode array has been advanced to the left ventricle via the retrograde aortic or the transseptal approach. Ventricular tachycardia is induced and immediately terminated after geometry creation. Presystolic critical circuit components and exit sites are readily identified; these localise critical, vulnerable components of the re-entrant circuit.19 Isolated diastolic potentials, presytolic areas, zones of slow conduction, and exit sites are also identified using virtual electrograms and isopotential maps during VT. Ablation of coronary VT not amenable to conventional ablation has been reported with success rates of approximately 75% at one year.
Non-contact mapping has also been used to guide ablation of idiopathic VT in both the right and left ventricles. Although focal and fascicular VTs are often amenable to conventional ablation approaches, when arrhythmia is non-sustained and infrequently present in the electrophysiology laboratory, conventional mapping may fail. We successfully ablated nine of 10 patients with difficult to treat right ventricular outflow tract VT, of whom seven had failed previous ablation and five had only transient arrhythmia in the electrophysiology laboratory. After a mean follow up of nine months, 78% of patients remained arrhythmia-free. Similar results have been observed in patients with previously failed ablation of idiopathic left VT.
Non-contact mapping strengths and limitations
Non-contact mapping's high density parallel data acquisition yields high resolution maps of the entire cardiac chamber from a single beat of tachycardia, enabling registration of transient or hypotensive arrhythmias. This also facilitates localisation of gaps in long linear lesions by pacing from both sides of the gap and rapidly remapping. Other useful features include radiation-free catheter navigation, re-visitation of points of interest, and cataloging ablation points on the three dimensional model. In patients with multiple or transient arrhythmias and no overt structural cardiac disease, non-contact mapping is the preferred approach.
Since isopotential maps are predominantly used, ventricular repolarisation must be distinguished from atrial depolarisation and diastolic ventricular activity. Early diastole may be challenging to map. Virtual electrogram quality deteriorates at a distance greater than 4 cm from the MEA, which at times may require MEA repositioning to acquire adequate isopotential maps. Lastly, substrate mapping (based on scar or diseased tissue) is limited with this technology at present.
OTHER ADJUNCTIVE TOOLS TO FACILITATE ABLATION
Catheter navigation systems have been developed that use low energy radiofrequency signals or ultrasound signals to localise catheters. These systems permit identification of points of interest in three dimensional space, cataloging of ablation sites, and re-navigation to sites of interest. However, one system does not create activation or isopotential maps, and does not integrate anatomy with physiology. Compared with the more complete mapping systems described above, its main advantage is reduced cost. Little clinical experience is available for the other system because of its recent introduction.
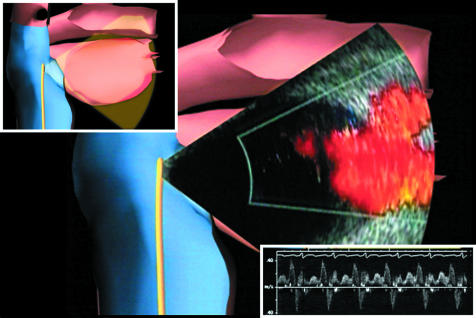
Cardiac imaging techniques, including intracardiac echocardiography, computed tomographic scanning, and magnetic resonance imaging have been used to plan or guide ablation. Several practical uses for intracardiac echo have emerged in the setting of electrophysiology procedures (fig 3). These include: assessment of catheter contact with cardiac tissues; determination of radiofrequency ablation lesions (by presence of “bubbles” during energy delivery and by tissue characterisation); determination of catheter location relative to cardiac structures (specifically useful in otherwise difficult to localise areas such as pulmonary veins); guidance of transseptal puncture, particularly in the setting of complex or unusual anatomy; facilitation of deployment of mapping or ablation systems such as pulmonary vein encircling devices, non-contact mapping systems, and basket technologies; evaluation of cardiac structures before and after intervention (such as cardiac valves and pulmonary veins); assessment of pulmonary vein anatomy, dimensions, and function via two dimensional anatomic imaging and Doppler physiologic measurements; and assessment of complications (for example, tamponade, electromechanical dissociation, or thrombus formation). Despite its many potential benefits, electrophysiology procedures can be performed in the absence of intracardiac echocardiography, and the role of this imaging modality is not yet defined.
Figure 3.
Phased array intracardiac echocardiography to image the left sided pulmonary veins with colour flow Doppler. Actual echocardiographic image is superimposed on computer model for orientation. Inset top left: computer graphic to depict ICE catheter position within the right atrium. Inset bottom right: pulse wave Doppler to quantify left superior pulmonary vein flow. This appears to be a sensitive indicator of venous stenosis during ablation. Reproduced from Darbar et al,2 with permission.
Novel mapping techniques for cardiac electrophysiology: key points.
▸ Advanced mapping systems are generally not required for catheter ablation of AVNRT, accessory pathway mediated tachycardia or AV junction ablation for rate control in atrial fibrillation because of the high success rate of conventional ablation approaches
▸ Advanced mapping systems are most useful for guiding ablation in haemodynamically unstable ventricular tachycardia, postsurgical macro-reentrant atrial arrhythmias, and transient arrhythmias.
▸ Advanced mapping systems reduce the need for ionising radiation (fluoroscopy) by means of non-fluoroscopic catheter navigation
▸ Electroanatomic mapping uses ultra low level magnetic fields for catheter localisation, and permits creation of complex three dimensional substrate (bipolar voltage) maps and activation maps for sustained/stable arrhythmias. Transient arrhythmias are not mapped
▸ Non-contact mapping uses low level electric fields for catheter localisation, and permits creation of complex three dimensional activation maps from a single complex, enabling mapping of transient arrhythmias. Substrate maps are not readily generated.
CONCLUSION
Conventional RF ablation has revolutionised the treatment of many supraventricular tachycardias and focal ventricular arrhythmias. As interest has turned to more complex arrhythmias, limitations of conventional treatment are being overcome with the introduction of sophisticated mapping systems that integrate three dimensional catheter localisation with sophisticated complex arrhythmia maps. This has added insight into mechanisms of arrhythmogenesis, and facilitated treatment of complex arrhythmias.
REFERENCES
- 1.Calkins H. Radiofrequency catheter ablation of supraventricular arrhythmias. Heart 2001;85:594–600. ▸ Recent comprehensive review of conventional catheter ablation of supraventricular tachycardia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darbar D, Olgin J, Miller J, et al. Localization of the origin of arrhythmias for ablation: from electrocardiography to advanced endocardial mapping systems. J Cardiovasc Electrophysiol 2001;12:1309–25. ▸ Recent review of advanced mapping systems and intracardiac imaging. [DOI] [PubMed] [Google Scholar]
- 3.Schalij MJ, van Rugge FP, Siezenga M, et al. Endocardial activation mapping of ventricular tachycardia in patients : first application of a 32-site bipolar mapping electrode catheter. Circulation 1998;98:2168–79. [DOI] [PubMed] [Google Scholar]
- 4.Kottkamp H, Hindricks G, Breithardt G, et al. Three-dimensional electromagnetic catheter technology: electroanatomical mapping of the right atrium and ablation of ectopic atrial tachycardia. J Cardiovasc Electrophysiol 1997;8:1332–7. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa H, Shah N, Matsudaira K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation 2001;103:699–709. ▸ Elegant paper describing the presence of multiple narrow channels as vulnerable ablation targets in patients with congenital heart disease and previous surgery, and use of electroanatomic mapping to localise them. [DOI] [PubMed] [Google Scholar]
- 6.Kottkamp H, Hügl B, Krauss B, et al. Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter : a prospective randomized study. Circulation 2000;102:2082–6. [DOI] [PubMed] [Google Scholar]
- 7.Shah D, Haissaguerre M, Jais P, et al. High-density mapping of activation through an incomplete isthmus ablation line. Circulation 1999;99:211–5. [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. ▸ Seminal paper describing the focal mechanism for atrial fibrillation, and the use of focal ablation for atrial fibrillation treatment. [DOI] [PubMed] [Google Scholar]
- 9.Pappone C, Oreto G, Lamberti F, et al. Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation 1999;100:1203–8. [DOI] [PubMed] [Google Scholar]
- 10.Ernst S, Schluter M, Ouyang F, et al. Modification of the substrate for maintenance of idiopathic human atrial fibrillation: efficacy of radiofrequency ablation using nonfluoroscopic catheter guidance. Circulation 1999;100:2085–92. [DOI] [PubMed] [Google Scholar]
- 11.Pappone C, Rosanio S, Oreto G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia. A new anatomic approach for curing atrial fibrillation. Circulation 2000;102:2619–28. [DOI] [PubMed] [Google Scholar]
- 12.Marchlinski F, Callans D, Gottlieb C, et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288–96. ▸ First paper to describe the use of substrate mapping to treat otherwise unmappable ventricular arrhythmias using electroanatomical mapping. [DOI] [PubMed] [Google Scholar]
- 13.Soejima K, Suzuki M, Maisel W, et al. Catheter ablation in patients with multiple unstable ventricular tachycardias after myocardial infarction. Short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation 2001;104:664–9. [DOI] [PubMed] [Google Scholar]
- 14.Gornick CC, Adler SW, Pederson B, et al. Validation of a new noncontact catheter system for electroanatomic mapping of left ventricular endocardium. Circulation 1999;99:829–35. ▸ Early article describing and validating non-contact mapping in a canine model and in vitro. System function described. [DOI] [PubMed] [Google Scholar]
- 15.Schneider MA, Ndrepepa G, Zrenner B, et al. Noncontact mapping-guided ablation of atrial flutter and enhanced-density mapping of the inferior vena cava-tricuspid annulus isthmus. Pacing Clin Electrophysiol 2001;24:1755–64. [DOI] [PubMed] [Google Scholar]
- 16.Betts T, Roberts P, Allen S, et al. Electrophysiological mapping and ablation of intra-atrial reentry tachycardia after Fontan surgery with the use of a noncontact mapping system. Circulation 2000;102:2094–9. [DOI] [PubMed] [Google Scholar]
- 17.Hindricks G, Kottkamp H. Simultaneous noncontact mapping of left atrium in patients with paroxysmal atrial fibrillation. Circulation 2001;104:297–303. [DOI] [PubMed] [Google Scholar]
- 18.Gasparini M, Mantica M, Coltorti F, et al. The use of advanced mapping systems to guide right linear lesions in paroxysmal atrial fibrillation. Eur Heart J 2001;3(suppl P):P41–6. [Google Scholar]
- 19.Schilling RJ, Peters NS, Davies DW. Feasibility of a noncontact catheter for endocardial mapping of human ventricular tachycardia. Circulation 1999;99:2543–52. ▸ The first article to describe the use of non-contact mapping for the endocardial mapping of human ventricular tachycardia. Validation of reconstructed electrograms and mapping of exit sites and diastolic components of circuit are described. [DOI] [PubMed] [Google Scholar]
- 20.Gepstein L, Hayam G, Ben-Haim SA. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation 1997;95:1611–22. [DOI] [PubMed] [Google Scholar]