Abstract
Aims: To investigate the hypothesis that changes in the ECG over time may be an important and readily available marker of prognostic value in patients with heart failure.
Methods: 112 elderly patients (81 men) with stable heart failure, a mean (SD) age of 73.3 (4.4) years, left ventricular ejection fraction 38 (17)%, and peak oxygen consumption 15.1 (4.7) ml/kg/min had ECG measurements on two occasions a minimum of 12 (5) months apart.
Results: During the subsequent follow up period (mean 27 (17) months) 45 patients died. QRS duration (p = 0.001) and heart rate (p = 0.03) at baseline were found by Cox proportional hazard method analysis to predict adverse outcomes in these patients. Of the changes in ECG parameters between the first and second visit, broadening of QRS duration (p = 0.001) predicted mortality. On Kaplan-Meier survival analysis, patients with < 5% change in QRS duration had fewer end points than patients with 5–20% change. A > 20% increase in QRS duration was associated with the worst prognosis. Progressive prolongation of QRS duration correlated closely with deterioration of LV systolic and diastolic function.
Conclusion: A single measurement of QRS duration has significant prognostic value in elderly patients with heart failure and the increase in QRS duration over time is an even better predictor of adverse out comes.
Keywords: heart failure, elderly, QRS duration, prognosis, electrocardiography, echocardiography
Heart failure remains associated with poor prognosis despite significant advances in the treatment and identification of multiple clinical and laboratory prognostic parameters.1,2 Little work has been done on the changes in these parameters over time and their prognostic value, especially in elderly patients with chronic heart failure. Established factors that determine prognosis include New York Heart Association (NYHA) classification,3 left ventricular ejection fraction (LVEF),4–6 and peak oxygen consumption.4,7,8 The prognostic importance of natriuretic peptides has also been established in chronic heart failure.9–11 Measurements of these parameters at one point in time has proved valuable in predicting prognosis; however, they are known to vary considerably during the course of the disease and treatment.12,13. NYHA classification is prone to subjective errors. Reproducibility of LVEF measurements varies depending on the techniques used.14,15 Peak oxygen consumption over time has been proved to be a better indicator of prognosis than absolute peak oxygen consumption, one of the powerful prognostic markers in patients with chronic heart failure. However, few hospitals have cardiopulmonary exercise testing facilities or have long term experience with it. We studied a simple, cheap, and universally available investigation: the ECG. Having previously shown that QRS duration is an important parameter in predicting prognosis in heart failure,16 here we assess the change over time in this and other ECG measurements and their prognostic value in a group of elderly patients with heart failure.
METHODS
One hundred and twelve patients (81 men), aged 68 years or above (consistent with the definition of aging thresholds of the population)17,18 with a clinically confirmed diagnosis of heart failure were recruited from a dedicated tertiary referral centre heart failure clinic. Patients had to have a 12 lead ECG recorded 12 months before the study. Patients were also required to have had their LVEF estimated by a transthoracic echocardiogram or a multigated nuclear imaging acquisition scan. Atrial fibrillation was an exclusion criterion to avoid high variability of QRS duration. Visit 1 was the time when the first ECG was recorded. The second ECG was recorded and studied after follow up period of 17 (5) months. After visit 2 patients were followed up for survival status for 27 (17) months. Patients on antiarrhythmic medications were not included in the study. Seventy nine (70%) patients were taking angiotensin converting enzyme inhibitors, 74 (66%) diuretics, 20 (18%) nitrates, 17 (15%) β blockers, 22 (19%) warfarin, and 65 (58%) aspirin. The end point was all cause cardiac mortality. Death was confirmed by the National Registry and survival was ascertained by hospital clinic attendance, general physician's records, and registration with the Office for National Statistics.
ECG measurements
Standard 12 lead ECGs were recorded with a Hewlett-Packard XLi Page Writer (Model M1700A, Hewlett-Packard, Andover, Massachusetts, USA) on a paper speed of 25 mm/s using calibration of 0.1 mV/mm. Parameters measured manually with electronic callipers (model no CD-6 CP, Mitutoyo UK Ltd, Andover, UK) were heart rate, RR interval, PR interval, QRS duration, QT interval, and corrected QT (QTc) interval using Bazzett's formula. These ECG parameters were measured in the V2 chest lead for standardisation. Patients who fulfilled the criteria for a classic bundle branch block were not excluded.
Echocardiograms
Echocardiograms were performed with a Hewlett-Packard Sonos 2000 echograph with a 2.5 MHz transducer interfaced to it. Left ventricular (LV) dimensions at end diastole (EDD) and at end systole (ESD) were taken from the M mode recording of the LV minor axis. End diastole was taken as the onset of the Q wave on the simultaneously recorded ECG and end systole as the onset of the aortic component of the second heart sound on the superimposed phonocardiogram. LVEF was estimated using the equation:
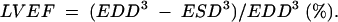 |
LV long axis amplitude was measured as previously described.19 Long axis recordings were made from the apical four chamber view with the cursor at the left and septal sites of the atrioventricular rings. LV isovolumic relaxation time (IVRT) was taken as the interval between the second heart sound and the onset of mitral cusp separation in early diastole. LV isovolumic contraction time was derived from the sum of the LV ejection time (the interval from the onset of the forward flow pulse across the aortic valve to the onset of the aortic closure artefact), LV filling time, and Doppler LV IVRT subtracted from the RR interval. LV filling measurements were taken from the transmitral pulsed Doppler recordings according to the recommendations of the American Society of Echocardiography.20,21 An ECG and phonocardiogram were recorded and superimposed on all M mode and Doppler traces. All records were obtained photographically at a paper speed of 100 mm/s.
Reproducibility of ECG and echocardiographic measurements
Reproducibility of ECG measurements was assessed by analysing two ECGs recorded one week apart in 20 subjects. Interobserver and intraobserver variability were also determined. The root mean square difference between two values was calculated and then divided by the mean of the absolute values. The coefficient of variability for interobserver variation for PR interval, QRS duration, and QT interval was found to be 3.1%, 4.0%, and 3.2%, respectively, and for intraobserver variation it was found to be 3.5%, 4.7%, and 3.0% respectively. The reproducibility of the echocardiographic overall LV performance in our laboratory has been previously described.22
Statistical analysis
Data were analysed using Statview 5.0 for Windows (SAS Institute, Cary, North Carolina, USA). Changes in ECG measurements over time with respect to baseline were studied using analysis of variance. Survival was analysed using the Cox proportional hazards method for continuous variables and the Kaplan-Meier method for nominal variables. A probability value of p < 0.05 was considered significant. Receiver operating characteristic curves were drawn to show the tradeoff between sensitivity and specificity for predicting an eventful outcome. Data are presented as mean (SD).
RESULTS
Table 1 shows ECG measurements for all patients, both survivors and non-survivors. Table 2 presents mean changes in these parameters over time and their p values. There were 34 patients in NYHA class I, 39 in class II, 30 in class III, and 9 in class IV. Follow up was completed for all patients within a mean of 27 (17) months. Mean LVEF for all patients was 38 (17)% at inclusion and 42 (18)% at follow up. At follow up, 45 (40%) patients had died.
Table 1.
Summary of electrocardiographic data for surviving and non-surviving patients at visits 1 and 2
| Visit 1 | Visit 2 | |||||
| Survivors | Non-survivors | p Value | Survivors | Non-survivors | p Value | |
| HR (beats/min) | 75 (15) | 79 (18) | 0.2 | 77 (16) | 81 (18) | 0.3 |
| PR (ms) | 178 (26) | 190 (50) | 0.1 | 174 (27) | 200 (4) | 0.002 |
| QRS (ms) | 106 (22) | 133 (25) | <0.0001 | 105 (17) | 150 (32) | <0.0001 |
| QT (ms) | 393 (46) | 395 (63) | 0.8 | 381 (43) | 391 (47) | 0.3 |
| QTc (ms) | 438 (47) | 453 (58) | 0.1 | 431 (52) | 454 (42) | 0.05 |
HR, heart rate; QTc, corrected QT interval.
Table 2.
Changes in ECG parameters over time (mean follow up 17 (5) months)
| All patients (n=112) | Survivors (n=67) | Non-survivors (n=45) | |||||||
| Visit 1 | Visit 2 | p Value | Visit 1 | Visit 2 | p Value | Visit 1 | Visit 2 | p Value | |
| HR (beats/min) | 77 (16) | 79 (17) | 0.05 | 75 (15) | 77 (16) | 0.04 | 79 (18) | 81 (18) | 0.56 |
| PR interval ( ms) | 181 (36) | 182 (35) | 0.8 | 178 (26) | 174 (27) | 0.59 | 190 (50) | 200 (41) | 0.43 |
| QRS duration (ms) | 115 (26) | 121 (32) | 0.0007 | 106 (22) | 105 (17) | 0.97 | 133 (25) | 150 (32) | <0.0001 |
| QT interval (ms) | 392 (52) | 383 (45) | 0.1 | 393 (46) | 381 (43) | 0.04 | 395 (63) | 391 (47) | 0.86 |
| QTc interval (ms) | 442 (52) | 439 (49) | 0.9 | 438 (47) | 432 (52) | 0.62 | 453 (58) | 454 (42) | 0.68 |
| RR interval (ms) | 815 (185) | 792 (170) | 0.1 | 847 (196) | 809 (145) | 0.01 | 749 (145) | 762 (210) | 0.61 |
Event-free patients versus patients with events
Heart rate and QT interval were not different between the two groups (77 (16) beats/min v 81 (18) beats/min, NS, and 381 (43) ms v 392 (47) ms, NS), respectively (table 1). Mean QRS duration at visit 1 in the event free group was 106 (22) ms v 133 (25) ms in patients with end points (p < 0.0001). The equivalent QRS durations at visit 2 were 105 (17) ms v 150 (32) ms in the two patient groups, respectively (p < 0.0001). The PR interval was shorter in event-free patients (174 (27) ms) than in those with end points (200 (41) ms, p = 0.002). Likewise, the QTc interval was modestly shorter in the survivors (431 (52) ms) than in those who died (454 (42) ms, p = 0.05). ECG measurements did not change in event-free patients over the course of 12 months whereas in patients who died QRS broadened by 17 ms over the same length of time.
Prognostic value of baseline and change in ECG measurements
Percentage change in QRS duration (p < 0.0001) was the most significant prognostic marker for adverse events (table 3). Allowing for this, no other variable was independently significant. Although PR interval was significantly longer in non-survivors than in survivors on the second visit, it could not predict mortality (table 2).
Table 3.
Univariate analysis by Cox proportional hazard method
| Baseline | p Value | Hazard ratio (95% CI) | % Change | p Value | Hazard ratio (95% CI) | |
| HR (beats/min) | 77 (16) | 0.03 | 1.026 (1.002 to 1.050) | 6 (20) | 0.3 | 0.987 (0.967 to 1.008) |
| PR interval (ms) | 181 (36) | 0.6 | 1.002 (0.993 to 1.012) | 2 (17.6) | 0.9 | 1.001 (0.981 to 1.022) |
| QRS duration (ms) | 115 (26) | 0.001 | 1.016 (1.006 to 1.027) | 6 (12) | <0.0001 | 1.038 (1.015 to 1.061) |
| QT interval (ms) | 392 (52) | 0.25 | 0.996 (0.989 to 1.003) | 1 (11.6) | 0.2 | 1.018 (0.992 to 1.044) |
| QTc interval (ms) | 442 (52) | 0.5 | 1.002 (0.996 to 1.009) | 1 (11) | 0.1 | 1.031 (0.994 to 1.069) |
| RR interval (ms) | 815 (185) | 0.01 | 0.996 (0.993 to 0.999) | 7 (27) | 0.5 | 1.003 (0.986 to 1.020) |
Difference in ECG measurements on the basis of aetiology
Of the 112 patients studied, 62% had ischaemic cardiomyopathy and 43 (38%) had idiopathic dilated cardiomyopathy. Heart rate was significantly lower in patients with ischaemic cardiomyopathy (73 (13) beats/min) than in those with idiopathic dilated cardiomyopathy (80 (14) beats/min, p = 0.03). There was no significant difference in PR interval, QRS duration, QT interval, or QTc interval. QRS duration at visit 1 did not have significant prognostic value.
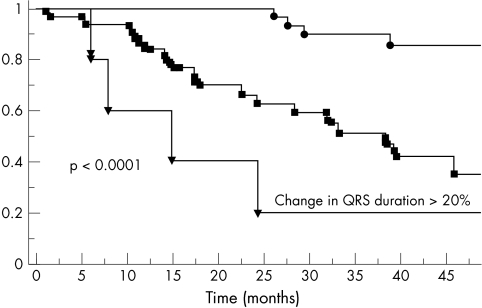
Kaplan-Meier curve for percentage change in QRS duration
Significantly more of the patients in the quartile with the highest percentage change in QRS duration died (fig 1). Percentage change in QRS duration was also a significant prognostic marker on univariate analysis (p < 0.0001), independent of the absolute change in QRS duration. Patients with a < 5% change in QRS duration had fewer end points than patients with 5–20% change. Patients with a > 20% increase in QRS duration had the worst prognosis. The results remained significant even after exclusion of two patients who developed recent left bundle branch block during the study period.
Figure 1.
Kaplan-Meier curves for percentage change in QRS duration over time.
Receiver operating characteristic curves for sensitivity of QRS value
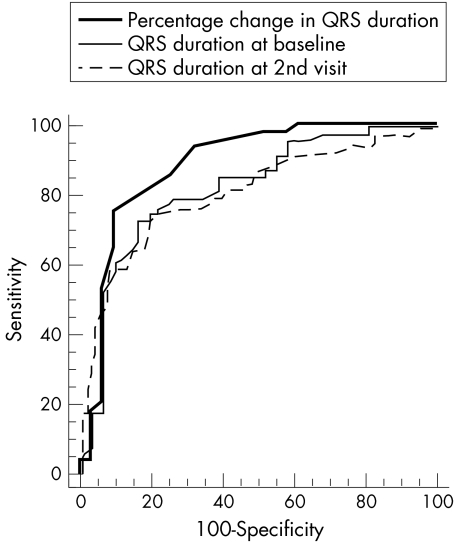
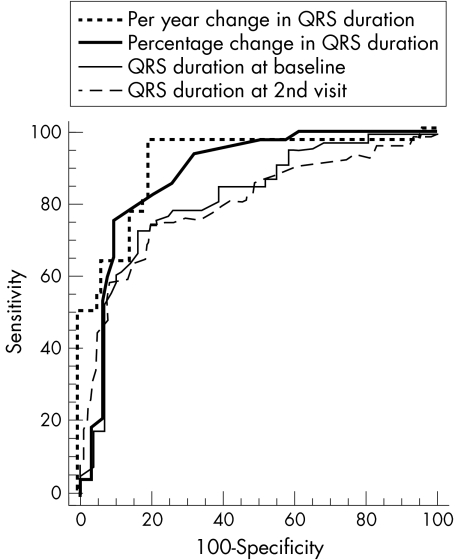
Receiver operating characteristic curves were compared for specificity and sensitivity. The area under the curve (AUC) for percentage change in QRS duration was 0.88 (0.04) (95% confidence interval (CI) 0.791 to 0.944) compared with a baseline of 0.79 (0.05) (95% CI 0.691 to 0.806) and QRS duration at the second visit of 0.77 (0.08) (95% CI 0.678 to 0.852; fig 22). This was significantly larger than at baseline (p = 0.002) and the second visit (p = 0.001). QRS duration was prolonged by 1.1 ms/year in survivors compared with 9.25 ms/year in non-survivors (p < 0.001; fig 33). This rate of change was 77% sensitive and 91.2% specific in predicting outcome. There was no statistical difference between the AUC for QRS duration at baseline and the AUC for QRS duration at the second visit.
Figure 2.
Comparison of receiver operating characteristic curves for percentage change in QRS duration with QRS duration at baseline and the second visit.
Figure 3.
Comparison of receiver operating characteristics curves for the rate of change for QRS duration per year.
QRS duration versus echocardiographic measurements
QRS duration > 120 ms was associated with a short IVRT (p < 0.001), increased EDD, and increased isovolumic contraction time (table 4). The same findings in addition to significantly poor LV long axis amplitude of motion (p < 0.01) were found in patients with events compared with those who did not have events. There was no significant change in LV dimensions, but IVRT fell significantly (p = 0.004) over time in patients with prolonged QRS duration (table 5). Furthermore, the change in QRS duration inversely correlated with the change in IVRT (r = −0.38, p = 0.04). It also correlated with ejection fraction, fractional shortening, and long axis amplitude, particularly with QRS duration broader than 120 ms, suggesting significant progressive LV disease (table 6).
Table 4.
Effect of QRS duration on echocardiographically derived parameters
| Echocardiographic parameter | QRS duration ≤120 ms | QRS duration >120 ms | p Value |
| LVESD | 4.8 (1.4) | 5.5 (1.1) | 0.1 |
| LVEDD | 5.9 (1.4) | 6.8 (0.9) | 0.03 |
| LA | 3.9 (0.7) | 4.2 (0.9) | 0.3 |
| IVRT | 75 (26) | 39 (32) | <0.0001 |
| LVCT | 86 (19) | 113 (54) | 0.03 |
IVRT, isovolumic relaxation time; LA, left atrial dimension; LVCT, isovolumic left ventricular contraction time; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter.
Table 5.
Difference in echocardiographic parameters in non-surviving and surviving patients with chronic heart failure
| Echocardiographic parameter | Non-survivors | Survivors | p Value |
| LVESD | 5.5 (1.4) | 4.5 (1.4) | 0.002 |
| LVEDD | 6.8 (1.6) | 5.9 (1.0) | 0.03 |
| IVRT | 24 (11) | 77 (26) | <0.0001 |
| LVET | 244 (42) | 278 (45) | 0.02 |
| LV amp | 1.0 (0.3) | 1.3 (0.5) | 0.02 |
| IVS amp | 0.7 (0.2) | 1.0 (0.4) | 0.01 |
IVS amp, interventricular septum long axis total amplitude; LVET, left ventricular ejection time; LV amp, left ventricular long axis total amplitude.
Table 6.
Correlation between QRS duration and echocardiographic parameters
| Echocardiographic parameter | Correlation coefficient | p Value |
| Visit 1 | ||
| IVRT | −0.40 | 0.0001 |
| Visit 2 | ||
| IVRT | −0.53 | <0.0001 |
| Change over time | ||
| LVEDD | 0.43 | 0.005 |
| LVESD | 0.57 | <0.0001 |
| FS | −0.73* | 0.0007 |
| LVEF | −0.46 | 0.002 |
| −0.80* | 0.0003 | |
| LVET | −0.64* | 0.01 |
| LV amp | −0.44 | 0.01 |
| −0.71* | 0.003 | |
| IVS amp | −0.57* | 0.01 |
| MR | 0.48 | 0.05 |
*In QRS duration >120 ms.
FS, fractional shortening; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
DISCUSSION
This study confirms previous findings in that absolute values of QRS duration correlate with prognosis in elderly patients with heart failure.16,23 In addition it shows that the relative change in QRS duration over time has a more sensitive prognostic value. QRS duration consistently increased by 17 ms over a period of 12 months in patients with end points compared with those with event-free outcome. Patients with a < 5% change in QRS duration over time had better survival than those with a change between 5–20%. The worst prognosis was seen in patients with a > 20% increase in QRS duration. Based on the Cox proportional hazards method and receiver operating characteristic curves, the percentage change in QRS duration (AUC = 0.88) was a stronger predictor and a more sensitive indicator of prognosis than absolute QRS values (AUC = 0.789) at any time point. Finally, changes in QRS duration correlated closely with changes in LV systolic function (ejection fraction, fractional shortening, and long axis excursion) and diastolic function (IVRT). These findings therefore confirm an important use of simply measured QRS duration as a predictor of outcome in patients with chronic heart failure.
Mechanisms of QRS broadening
A QRS duration > 120 ms24–27 is widely used as a diagnostic criterion for bundle branch block, although the literature lacks convincing evidence to support this threshold. The earliest citation of the value 120 ms can be traced to 1947, when Wilson and colleagues27 stated that “in complete bundle branch block the QRS duration measures at least three small square (i.e. 120 milliseconds)”. In the absence of definitive normal values for QRS in different age groups, change in QRS duration with time seems to provide an accurate assessment of the change in conduction time. This principle has not only proved to be sensitive in identifying patients with conduction delay—that is, progressive disease—but also offered a prognostic tool that can be used in the follow up of patients with heart failure. The increase in QRS duration over time also correlated with markers of deterioration of ventricular systolic function and increase in filling pressures. These electrical and functional findings are closely related to each other. Progressive LV disease eventually results in increased myocardial stiffness and a rise in diastolic pressures. The resulting increase in LV filling pressures and consequent ischaemia of the subendocardium predispose the latter to conduction “depolarisation” delay. A longstanding unstable condition such as this may result in subendocardial fibrosis and permanent conduction disturbance. Thus, in dilated and dysfunctioning ventricles, progressive broadening of the QRS complex can be taken as a marker of perpetual worsening of ventricular disease. This is supported by the close correlation found in this study between the two.
The QRS complex is known for specific disturbances in ventricular disease: a voltage increase in ventricular hypertrophy and deep Q wave with fibrosis and scarring complicating myocardial infarction. Even the absence of a normal septal Q wave has long been understood to be a marker of subendocardial fibrosis.28 Although knowledge of the S wave itself is scanty, the overall duration of the QRS complex seems to correlate closely with subendocardial function, particularly in the presence of coronary artery disease. This has been shown to broaden during stress as the myocardium becomes ischaemic.29 The opposite has been shown after release of the fixed ventricular afterload in patients with aortic stenosis and severe ventricular disease.30 Therefore, it seems that QRS duration can be used as a sensitive marker for overall ventricular function and possibly overloading.
The value of the change in QRS duration over time in predicting clinical outcome in patients with chronic heart failure is complementary to our previous report on patients with dilated cardiomyopathy.18 In them, QRS duration did not significantly change in stable patients compared with patients with events of either death or pacemaker implantation. In fact in the two studies, a 20% increase in QRS duration over a period of 12 months was associated with consistent end points. In the present study, in addition, such an electrical delay correlated with markers of deterioration of ventricular function and increase in filling pressures. This confirms a close relation between electrical, haemodynamic components of cardiac function and clinical outcome. These findings are also in agreement with our previous report in showing a fall in IVRT with poor outcome in patients with heart failure.31,32 Furthermore, they are consistent with other reports in showing that a fall in ejection fraction, exercise tolerance, and transmitral E wave deceleration time has a deleterious effect on clinical outcome in patients with heart failure.5,12,33,34 Finally, progressive prolongation of the P wave has been found to predict the occurrence of atrial arrhythmias (atrial fibrillation and flutter) and deterioration of function.35 Similarly, the progressive increase in QRS duration over time may lead to ventricular arrhythmia resulting in complete mechanical standstill.
Clinical implications
A simple measure of the change in QRS duration over time can easily be used in all heart failure clinics as well as in patients on the waiting list for heart transplantation. A rapidly prolonging QRS duration should favour early intervention, if not with a transplantation, then by an assist device or a pacemaker. Theoretically, a fall in QRS duration with time may suggest an improvement of ventricular function and hence outcome.
Limitations
This study has limitations. The study encompassed an aetiologically heterogeneous group of patients with idiopathic dilated or ischaemic cardiomyopathy, in whom right ventricular function plays an important part.36 We did not measure LV end diastolic pressures but relied on its non-invasive estimation—that is, short IVRT and E wave deceleration time. The majority of these patients had some degree of functional mitral regurgitation but we did not find a significant difference in either its incidence or severity between groups to explain the findings.
Conclusion
Twelve lead ECG recording appears to be an important tool for clinical follow up of patients with chronic heart failure. A progressive increase in QRS duration predicts significant deterioration of ventricular function and a rise in filling pressures, whereas a stable QRS duration signifies maintained LV function and behaviour. Since the combination of electrical and functional deterioration predicts poor outcome, applying these findings in patients with chronic heart failure may optimise further management accordingly.
Supplementary Material
Abbreviations
AUC, area under the curve
EDD, end diastolic diameter
ESD, end systolic diameter
IVRT, isovolumic relaxation time
LV, left ventricular
LVEF, left ventricular ejection fraction
NYHA, New York Heart Association
QTc, corrected QT interval
REFERENCES
- 1.Franciosa JA. Epidemiologic patterns, clinical evaluation, and long-term prognosis in chronic congestive heart failure. Am J Med 1986;80:14–21. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JR, Schwartz JS, Sutton MS, et al. Prognosis in severe heart failure: relation to haemodynamic measurements and ventricular ectopic activity. J Am Coll Cardiol 1983;2:403–10. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson LW. Selection and management of patients for cardiac transplantation. Curr Opin Cardiol 1994;9:315–25. [DOI] [PubMed] [Google Scholar]
- 4.Parameshwar J, Keegan J, Sparrow J, et al. Predictors of prognosis in severe chronic heart failure. Am Heart J 1992;123:421–6. [DOI] [PubMed] [Google Scholar]
- 5.Cintron G, Johnson G, Francis G, et al. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation 1993;87:VI17–23. [PubMed] [Google Scholar]
- 6.Keogh AM, Baron DW, Hickie JB. Prognostic guides in patients with idiopathic or ischaemic dilated cardiomyopathy assessed for cardiac transplantation. Am J Cardiol 1990;65:903–8. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart 2000;83:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin BP, Shah PK, Ferguson J, et al. Incremental prognostic value of exercise hemodynamic variables in chronic congestive heart failure secondary to coronary artery disease or to dilated cardiomyopathy. Am J Cardiol 1991;67:848–53. [DOI] [PubMed] [Google Scholar]
- 9.Cowie MR, Mosterd A, Wood DA, et al. The epidemiology of heart failure. Eur Heart J 1997;18:208–25. [DOI] [PubMed] [Google Scholar]
- 10.Madsen BK, Keller N, Christiansen E, et al. Prognostic value of plasma catecholamines, plasma renin activity, and plasma atrial natriuretic peptide at rest and during exercise in congestive heart failure: comparison with clinical evaluation, ejection fraction, and exercise capacity. J Card Fail 1995;1:207–16. [DOI] [PubMed] [Google Scholar]
- 11.de-Groote P, Millaire A, Pigny P, et al. Plasma levels of atrial natriuretic peptide at peak exercise: a prognostic marker of cardiovascular related death and heart transplantation in patients with moderate congestive heart failure. J Heart Lung Transplant 1997;16:956–63. [PubMed] [Google Scholar]
- 12.Stevenson LW, Steimle AE, Fanorow G, et al. Improvement in exercise capacity of candidates awaiting heart transplantation. J Am Coll Cardiol 1995;25:163–70. [DOI] [PubMed] [Google Scholar]
- 13.Gullestad L, Myers J, Ross H, et al. Serial exrecise testing and prognosis in selected patients considered for cardiac transplantation. Am Heart J 1998;135:221–9. [DOI] [PubMed] [Google Scholar]
- 14.Cowburn PJ, Cleland JG, Coats AJ, et al. Risk stratification in chronic heart failure. Eur Heart J 1998;19:696–710. [DOI] [PubMed] [Google Scholar]
- 15.Ray SG, Metcalfe MJ, Oldroyd KG, et al. Do radionuclide and echocardiographic techniques give a universal cut off value for left ventricular ejection fraction that can be used to select patients for treatment with ACE inhibitors after myocardial infarction? Br Heart J 1995;73:466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamim W, Francis DP, Yousufuddin M, et al. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol 1999;70:171–8. [DOI] [PubMed] [Google Scholar]
- 17.McCreadie C, Tinker A. Abuse of elderly people in the domestic settings: a UK perspective. Age Ageing 1993;22:65–9. [DOI] [PubMed] [Google Scholar]
- 18.Seigel JS. Recent and prospective demographic trends for the elderly population and some implications for health care. In: Second conference on the epidemiology of aging, Bethesda, MD, March 28–29, 1977. Washington DC: Government Printing Office, 1988:289–315.
- 19.Henein MY, Das SK, O'Sullivan C, et al. Effect of acute alterations in after-load on left ventricular function in patients with combined coronary artery and peripheral vascular disease. Heart 1996;75:151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahn DJ, DeMaria AN, Kisslo JA, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- 22.Henein MY, Amadi A, O'Sullivan CA, et al. ACE inhibitors unmask inco-ordinate diastolic wall motion in restrictive left ventricular disease. Heart 1996;76:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao HB, Roy C, Gibson DG. Nature of ventricular activation in patients with dilated cardiomyopathy: evidence for bilateral bundle branch block. Br Heart J 1994;72:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowlands DJ. Intraventricular conduction disturbances. In: Clinical cardiology. London: Gower Medical Publishing, 1991:110–43.
- 25.The Criteria Committee of the New York Heart Association Inc. Nomenclature and criteria for diagnosis of the heart and blood vessels. New York: New York Heart Association, Inc, 1953.
- 26.Willems JL, Robesl de Medina EO, Bernard R, et al. Criteria for intraventricular conduction disturbances and pre-excitation. World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol 1985;5:1261–75. [DOI] [PubMed] [Google Scholar]
- 27.Wilson FN, Rosenbaum FF, Johnston FD. Interpretation of the ventricular complex of the electrocardiogram. In: Dock W, Snapper I, eds. Advances in internal medicine. New York: Interscience Publishers, Inc, 1947:1–63.
- 28.Burch GE, DePasquale N. A study at autopsy of the relation of absence of the Q waves in leads I, avL, V5 and V6 to septal fibrosis. Am Heart J 1960;60:336–40. [PubMed] [Google Scholar]
- 29.O'Sullivan CA, Henein MY, Sutton R, et al. Abnormal ventricular activation and repolarisation during dobutamine stress echocardiography in coronary artery disease. Heart 1998;79:468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collinson J, Henein MY, Flather M, et al. Valve replacement for aortic stenosis in patients with poor left ventricular function: comparison of early changes with stented and stentless valves. Circulation 1999;100(19 suppl):II1–5. [DOI] [PubMed] [Google Scholar]
- 31.Florea VG, Henein MY, Anker SD, et al. Relation of changes over time in ventricular size and function to those in exercise capacity in patients with chronic heart failure. Am Heart J 2000;139:913–7. [DOI] [PubMed] [Google Scholar]
- 32.Florea VG, Henein MY, Anker SD, et al. Prognostic value of changes over time in exercise capacity and echocardiographic measurements in patients with chronic heart failure. Eur Heart J 2000;21:146–53. [DOI] [PubMed] [Google Scholar]
- 33.Levine TB, Levine AB, Goldberg D, et al. Reversal of end-stage heart failure is predicted by long-term therapeutic response rather than initial hemodynamic and neurohormonal profile. J Heart Lung Transplant 1996;15:297–303. [PubMed] [Google Scholar]
- 34.Szlachic J, Massie BM, Kramer BL, et al. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 1985;55:1037–42. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Xiao HB, Henein MY, et al. Progressive ECG changes before the onset of atrial flutter in adult congenital heart disease patients. Heart 2001;85:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb-Peploe KM, Henein MY, Coats AJ, et al. Echo derived variables predicting exercise tolerance in patients with dilated and poorly functioning left ventricle. Heart 1998;80:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.