Junctional ectopic tachycardia (JET) is a rare arrhythmia presenting either as a primary form1 or more often as a transient phenomenon immediately after heart surgery.2 Congenital JET usually occurs in the first six months of life as a persistent arrhythmia, associated in up to 60% of cases with cardiomegaly and/or heart failure.1
Congenital JET has been described in sporadic case reports or larger multicentre populations. The purpose of this study is to examine retrospectively the clinical presentation and outcome of a cohort of nine patients with congenital JET referred during a 20 year period time to a single unit and all medically treated.
METHODS
Nine patients (two male, seven female) suffering from persistent sustained JET were referred to our hospital between 1980 and 1999. All the patients underwent a complete medical history, physical examination, 12 lead ECG, 24 hour Holter recording, and echocardiogram.
The diagnosis of JET was based on electrocardiographic evidence of a narrow complex tachycardia and atrioventricular dissociation.
All the patients underwent medical treatment according to the following regimen:
Digoxin (5–10 μg/kg/day orally) was given for controlling the symptoms of cardiac failure, in the presence of cardiomegaly and/or decreased left ventricular function
Propafenone (10–15 mg/kg/day orally) was used in all patients, alone or in combination with digoxin, as first step of treatment
Amiodarone (loading dose 12–27 mg/kg/day for seven days, followed by a maintenance dose of 5–12 mg/kg/day orally) was the next drug administered if propafenone proved ineffective after four days of treatment.
A Ic antiarrhythmic drug (propafenone or flecainide) was administered in combination with amiodarone when the latter proved ineffective alone in controlling the arrhythmia. This combination of drugs was also used in an attempt to reduce the dose of amiodarone, and prevent signs of drug toxicity.
The doses of amiodarone and propafenone, used in combination, were 5 mg/kg/day and 5–10 mg/kg/day, respectively. Flecainide was used in combination with amiodarone at a dose of 2–5 mg/kg/day orally.
Repeat ECGs and 24 hour ambulatory recordings assessed the results of treatment.
Treatment success was defined as a stable decrease, on ECG Holter, in the mean heart rate of ≤ 140 beats per minute (bpm), while partial success was defined as a decrease in the rate of tachycardia (usually mean heart rate ≤ 170 bpm) that resulted in alleviation of symptoms or signs of heart failure. Patients were discharged in the presence of total or partial treatment success. They were seen in our outpatient clinic at two weeks, one, three, and six months after the discharge, and at four month intervals thereafter. However, follow up time was modified in case of impaired clinical status. Assessment at follow up visits included 12 lead ECG, 24 hour Holter, echocardiogram, and blood analyses.
RESULTS
Table 1 shows patient characteristics, JET features, and drug treatment.
Table 1.
Patient characteristics, junctional ectopic tachycardia features, and drug treatment
| Patient | Sex | JET onset | Heart rate at presentation (bpm) | Heart rate at discharge (bpm) | Familiarity | Heart failure | Decreased SF/EF | Treatment | Follow up |
| 1 | F | 6 months | 190 | 100 | – | – | + | DG + PF | 14.6 years |
| 2 | F | 4 months | 170 | 110 | – | – | – | PF | 7.1 years |
| 3 | F | 6 months | 240 | 170 | + | + | + | DG+AM | 12.5 years |
| 4 | F | 2 months | 170 | 120 | + | – | – | AM+PF | 8.2 years |
| 5 | M | 5 months | 170 | 110 | – | – | – | PF | 21.5 years |
| 6 | F | 3 months | 300 | 130 | + | + | + | DG+AM+PF | 18.8 years |
| 7 | M | 2 months | 270 | 130 | + | + | + | DG+AM+PF | 16.5 years |
| 8 | F | 1 month | 240 | 140 | – | – | + | DG+AM+FL | 2.6 years |
| 9 | F | 6 months | 300 | 120 | + | + | + | DG+AM+FL | 10.5 years |
AM, amiodarone; DG, digoxin; EF, ejection fraction; FL, flecainide; JET, junctional ectopic tachycardia; PF, propafenone; SF, shortening fraction.
A family history of JET was positive in five of the nine patients. In particular, three members of the same family (two sisters, one cousin) entered our study. Two patients had congenital heart disease: an ostium secundum atrial defect and a small ventricular septal defect.
Digoxin was given in six patients to control the symptoms of cardiac failure or in the presence of decreased left ventricular function.
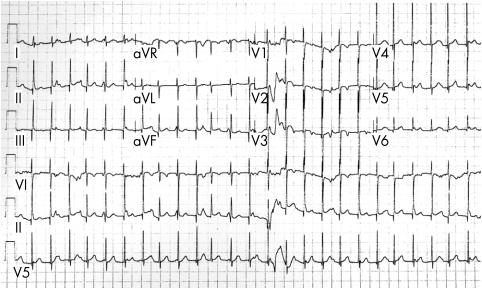
Propafenone was initially given to all the patients. In the presence of clinical or instrumental signs of impaired ventricular function (six patients) the drug was given in combination with digoxin. In three patients propafenone slowed the ventricular rate (177 bpm v 107 bpm) (fig 1). In six patients the drug was completely ineffective.
Figure 1.
Twelve lead ECG in 4 month old patient treated with propafenone (junctional ectopic tachycardia with a heart rate of 157 bpm).
Amiodarone alone was the next drug used in the six patients who did not respond to propafenone. The drug proved successful in two patients, partially successful in one patient, and failed in three patients.
The combination of amiodarone and propafenone proved successful in treating two patients and was partially successful in one patient.
During the follow up, the combination of amiodarone and flecainide was successfully used in two patients initially treated with high dose amiodarone alone (12 mg/kg/day), enabling a reduction in the dose of amiodarone.
Systemic side effects did not occur during treatment in all the patients, and ventricular function did not deteriorate. No patients underwent His bundle catheter ablation.
Two patients treated respectively with the combination of amiodarone + propafenone and amiodarone + flecainide showed, during follow up, non-sustained symptomatic ventricular tachycardia. In both the cases the ventricular tachycardia disappeared, halving the Ic antiarrhythmic drug dose and increasing the amiodarone dose.
Follow up periods range from 2.6–21 years. All the patients are still being treated, as all attempts to stop medical treatment resulted in an uncontrollable tachycardia.
DISCUSSION
JET has been reported in older children and adults, and as a transient phenomenon immediately after surgery for congenital heart defects.2 However, the outcome and prognosis of the acquired form is very different from the congenital form, and patients presenting with JET in childhood or adolescence have been excluded from the present study.
Clinical features
The occurrence of the clinical presentation of congenital JET usually ranges from birth to 4 weeks of age. Sporadic cases of history of intrauterine tachycardia have been reported in patients who showed overt JET at birth.1
In our series, a high proportion of the patients presented, at the time of referral, with echocardiographic evidence of impaired left ventricular function (6/9) and clinical signs of congestive heart failure (4/9). The age of tachycardia presentation was not related to the occurrence of congestive heart failure or impaired left ventricular function (3.7 months in patients with normal function v 4 months in patients with impaired function). Clinical status seemed to be related to the ventricular rate at presentation (170 bpm in patients with normal ventricular function v 257 bpm in patients with impaired function).
A family history of JET has been recognised in 55.6% of our patients, similar to previous studies reporting a 50% incidence of the familial form.1 Particularly, three members of the same family (two sisters, one cousin) entered our study.
Death
Congenital JET has a high mortality rate, up to 34%.1 In our series no deaths were recorded. The exact mechanism related to death in congenital JET is still unclear. Sporadic cases of sudden death have been attributed to a dramatic evolution to paroxysmal complete atrioventricular block.1,3 It has been suggested that the same His bundle degenerative process that supports JET could later extend to cause atrioventricular block. However, in some cases the potential proarrhythmic effects of the drugs used to control the arrhythmia could not be excluded as a cause.4 In our series, two patients developed symptomatic non-sustained ventricular tachycardia as a spontaneous desynchronisation of JET, caused by the proarrhythmic effect of the drug combination. This observation outlines the necessity for accurate monitoring of drug action during long term treatment, especially when drug combinations are used.
Treatment
Treatment is indicated in infants with symptoms, reduced ventricular function or rapid heart rate. The most appropriate management of infants with slow JET (h 150 bpm) without symptoms is debatable. However, the importance of monitoring these asymptomatic patients accurately is undoubted. Our policy is to use digoxin only in controlling the symptoms of cardiac failure, giving antiarrhythmic drugs to control the ventricular rate of arrhythmia. Digoxin use has not been shown to be completely safe in patients with JET. Patients affected by congenital JET and severe cardiac failure have been reported to develop ventricular fibrillation or faster tachycardia (up to 400 bpm) during progressive digoxin loading.1
Despite the excellent results presented in previous work,5 in our series propafenone, used alone, resulted in effectively preventing or controlling tachycardia in three patients only, especially in those patients with lower heart rate (mean rate during tachycardia 170 bpm). According to a previous report,6 amiodarone was substantially successful within the first five days of treatment, without any complications during follow up. Furthermore, we noted that the combination of amiodarone and a Ic antiarrhythmic drug could be used to reduce the dose of amiodarone, with excellent results.
In conclusion, there are various medical treatments for JET. A pharmacological approach should be regarded as the first line therapeutic option. Physicians should not be discouraged by initial failure, because “true” drug refractory JET is very rare. In those patients who fail to respond to a single drug regimen, the addition of an antiarrhythmic agent with different electrophysiological effects may prove effective.
Acknowledgments
Supported by Institute of Cardio-Pulmonary Researches, Monaldi Hospital, Naples, and financed by the Operative Programme Plan Critical Neonatal Congenital Heart Disease (CCCN5) BOO6 of the University and Scientific Research Ministry (MURST) and the European Community (711/1998).
REFERENCES
- 1.Villain E, Vetter VL, Garcia JM, et al. Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation 1990;81:1544–9. [DOI] [PubMed] [Google Scholar]
- 2.Walsh EP, Saul P, Sholler GF, et al. Evaluation of a staged treatment protocol for rapid automatic junctional tachycardia after operation for congenital heart disease. J Am Coll Cardiol 1997;29:1046–53. [DOI] [PubMed] [Google Scholar]
- 3.Henneveld H, Hutter P, Bink-Boelkens M, et al. Junctional ectopic tachycardia evolving into complete heart block. Heart 1998;80:627–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap SC, Hoomtje T, Sreeram N. Polymorphic ventricular tachycardia after use of intravenous amiodarone for postoperative junctional tachycardia. Int J Cardiol 2000;76:245–7. [DOI] [PubMed] [Google Scholar]
- 5.Paul T, Reimer A, Janousek J, et al. Efficacy and safety of propafenone in congenital junctional ectopic tachycardia. J Am Coll Cardiol 1992;20:911–14. [DOI] [PubMed] [Google Scholar]
- 6.Raja P, Hawker RE, Chikitpinyo A, et al. Amiodarone management of junctional ectopic tachycardia after cardiac surgery in children. Br Heart J 1994;72:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]