Abstract
Objective: To investigate ventricular sympathetic innervation in patients with sick sinus syndrome and to detect regional deterioration of adrenergic innervation caused by asynchronous ventricular activation from right ventricular pacing.
Design: Prospective controlled study.
Setting: Tertiary cardiac referral centre.
Patients: 22 patients with sick sinus syndrome and indications for permanent dual chamber pacing; 20 healthy individuals as controls.
Interventions: All patients underwent myocardial imaging with planar and single photon emission computed tomography (SPECT) after an intravenous infusion of 5 mCi 123I-meta-iodobenzylguanidine (123I-MIBG) before and after pacemaker implantation. A SPECT thallium201 myocardial study was done during the same week as the 123I-MIBG study in all patients.
Main outcome measures: The heart to mediastinum (H/M) ratio and washout rate were calculated during the 123I-MIBG study to assess the global cardiac sympathetic activity; the aim of the SPECT study was to investigate the regional distribution of adrenergic innervation.
Results: The H/M ratio was significantly smaller in the patients with sick sinus syndrome than in the controls (p < 0.001). In sick sinus syndrome there were regional adrenergic innervation defects, mostly in the inferior and apical walls. After a medium term pacing period, a redistribution of 123I-MIBG uptake was detected, with deterioration of adrenergic innervation in the inferior, apical, and posterior walls. The thallium201 myocardial perfusion study showed no change after three months of permanent pacing.
Conclusions: Patients with sick sinus syndrome have global and regional disturbances of the adrenergic innervation of the left ventricular myocardium. These seem to deteriorate as a result of asynchronous electrical activation. The clinical significance of this finding requires further investigation.
Keywords: pacing, sick sinus syndrome, adrenergic innervation
Sick sinus syndrome, in which there is progressive dysfunction of the sinus node, is one of the most common indications for a permanent pacemaker. The sites that are normally used for electrode implantation in permanent cardiac pacing are the high right atrium and the apex of the right ventricle. However, it has been shown that pacing through the right ventricular apex is associated with alterations in the contraction and relaxation pattern of the left ventricle,1–3 remodelling, and histological abnormalities in the left ventricular myocardium.4–7 Recently, there have been indications that long term permanent pacing is related to abnormalities of regional perfusion and adrenergic innervation.8,9 However, there is a lack of comparable data on these perfusion and innervation disturbances before and after pacemaker implantation, so the net real effect of pacing has not been clarified. Patients with sick sinus syndrome have rhythm disturbances that may be associated with alterations in cardiac autonomic tone affecting myocardial adrenergic innervation, so it is important to know whether any adrenergic innervation defects found in paced patients were present before the start of pacing.
In our study, we evaluated the adrenergic innervation of the left ventricle in patients with sick sinus syndrome using the radioionated noradrenaline (norepinephrine) analogue, 123I-meta-iodobenzylguanidine (123I-MIBG) as an imaging marker.10 We also assessed the alterations in regional myocardial adrenergic innervation in these patients after the implantation of a permanent dual chamber pacemaker, in order to examine the effect of the asynchronous ventricular activation stimulus.
METHODS
Patient population
We studied 22 consecutive patients (12 men, 10 women; mean (SD) age, 65.05 (6.11) years) with sick sinus syndrome and indications for dual chamber, rate adaptive (DDDR) pacemaker implantation. The diagnosis was suggested by the presence of the following:
symptomatic persistent spontaneous sinus bradycardia not caused by drugs
sinus arrest or exit block
combinations of sinoatrial and atrioventricular conduction disturbances
alteration between paroxysms of atrial tachyarrhythmias and periods of slow atrial and ventricular rates, documented by ECG or 24 hour Holter recordings.
Patients with diabetes mellitus and arterial hypertension, a history of coronary artery disease, or clinical indications of organic heart disease or heart failure of any type were excluded.
As controls, we also studied 20 healthy individuals with a similar sex and age distribution. They had no signs of heart disease and their clinical, echocardiographic, and thallium201 scintigraphic findings were normal.
A dual chamber permanent pacemaker with a Holter recording system was implanted in each patient, with the atrial lead placed in the right atrial appendage and the ventricular lead in the right ventricular apex. All pacemakers were capable of rate adaptation of atrioventricular delay, and they were programmed to achieve the longest delay that maintained the maximum QRS duration observed under VVI pacing, so that ventricular activation would be fully paced. The patients underwent pacemaker interrogation to determine the proportion of paced ventricular systoles three months after the implantation, and 24 hour Holter monitoring (Elatec V3.03B; Ela Medical, La Boursidiere, Le Plessis-Robinson, France) for assessment of ventricular activation patterns. They also underwent an echocardiographic examination (Sonos 2500; Hewlett Packard Inc, Andover, Massachusetts, USA) to evaluate left ventricular structure and function.
Before and three months after pacemaker implantation, all patients completed a myocardial thallium201 scintigraphic perfusion study and an 123I-MIBG myocardial scintigraphic study during the same week to assess adrenergic innervation. The control group also underwent thallium201 and 123I-MIBG myocardial scintigraphic examinations. None of the subjects was receiving drug treatment or had any clinical condition that has been shown to affect 123I-MIBG uptake. Subjects with thallium201 perfusion defects were referred for coronary angiography. Patients with coronary artery stenosis of more than 50% were excluded from the study. We also excluded obese patients and women with large breasts.
All subjects provided their written informed consent for inclusion in the study. The hospital's ethics committee approved the protocol.
Imaging protocols
Dipyridamole-thallium201 myocardial scintigraphy
All subjects were fasted overnight. They had a four minute intravenous infusion of dipyridamole, 0.56 mg/kg body weight, and three minutes later 3 mCi of thallium201 was injected intravenously. A 12 lead ECG was recorded before testing and at one minute intervals for a minimum of 10 minutes after the dipyridamole infusion was started.
Any therapeutic agents containing methylxanthine were discontinued for a minimum of 48 hours before dipyridamole administration; no caffeine containing beverages were consumed within the 24 hours of the test.
Imaging was achieved with a rotating, dual head gamma camera (GE Medical Systems, Milwaukee, Wisconsin, USA) 10 minutes and four hours after the injection. Thirty two projections (40 seconds each) were obtained over a 180° arc, beginning with the 45° left posterior oblique and finishing with the 45° right anterior oblique view. All images were stored using a 64 × 64 matrix. Transaxial, sagittal, and oblique tomograms were obtained using a nuclear medicine computer (software: GENIE v. 2.5H, 99224, rev. 137).
123I-MIBG scintigraphy
On the day of the 123I-MIBG scintigraphy all patients and controls were instructed to fast for six hours. Lugol's solution (1 ml) was given orally two hours before a slow intravenous injection of 5 mCi 123I-MIBG (Mallinckrodt Inc, St Louis, Missouri, USA; specific activity 74 MBq/mg).
At 10 minutes, one hour, and four hours after the tracer injection, a 10 minute static acquisition was performed in the anterior view of the chest, using a General Electric (GE Medical Systems) large field of view, single head gamma camera fitted with a low energy, all purpose, parallel hole collimator. A 20% energy window centred on 157 keV and a 128 × 128 matrix size were used. After the delay planar image, single photon emission computed tomography (SPECT) was done using a dual head gamma camera (GE Medical Systems). Thirty two projections (50 seconds each) were obtained over a 180° arc, from left posterior oblique to right anterior oblique, and the images were stored using a 64 × 64 matrix. Transaxial, sagittal, and oblique tomograms were obtained using a nuclear medicine computer (software: GENIE v. 2.5H, 99224, rev. 137).
Cardiac uptake was quantified in all planar views. A 7 × 7 pixel region of interest was drawn over the cardiac region and another 7 × 7 region of interest over the upper mediastinum area. The heart to mediastinum (H/M) activity ratio, introduced by Merlet and colleagues,11 was then computed to quantify cardiac 123I-MIBG accumulation. Two independent observers measured the H/M ratio, and the average of the two measurements was taken as the datum. The clearance rate from the myocardium (washout rate) was calculated as follows:
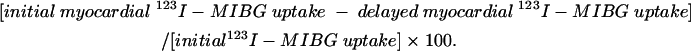 |
In SPECT imaging the left ventricular myocardium was divided into six segments. 123I-MIBG scintigrams were examined by two independent experts—with no knowledge of the patients' clinical and angiographic status—for the presence of any abnormalities. A four point scoring system was used to provide a defect score for visual interpretation of the 123I-MIBG and thallium201 scintigraphy in the six segments of left ventricular myocardium, as follows: 3, absence of detectable tracer in a segment, or severe reduction of radioisotope uptake; 2, moderately reduced uptake; 1, mildly reduced uptake; 0, normal radioisotope uptake.
Statistical analysis
Continuous data are summarised as mean (SD). Continuous variables were compared between the two groups with an independent samples t test. Changes in 123I-MIBG scores before and after pacing were evaluated using paired samples t tests. Changes in the time course of the ratio between the two groups were assessed with a repeated measures analysis of variance (ANOVA) model with two repeated and one between factors. One of the repeated factors had three levels (at 10 minutes, 60 minutes, and four hours) and the other had two (before–after). Bonferroni adjustments were made in multiple comparisons. The criterion for significance was set at 5%.
The interobserver variability for the H/M ratio was very low, the 95% confidence interval for difference being −0.043 to 0.043 for prepacing values, and −0.108 to 0.123 for values after pacing.
RESULTS
Five of the 22 patients initially included in the study, and none of the control group, had perfusion defects on thallium201 myocardial scintigraphy. Two of the patients had significant coronary artery stenosis (more than 50%) in one or more vessels and were excluded from the study. The clinical characteristics of the remaining patients and controls are shown in table 1, together with the main results. All patients had ventricular pacing throughout most of the 24 hour monitoring period (completely paced ventricular beats in 96.5 (18.5)%); the interrogation of the pacemakers revealed ventricular pacing in 95.9 (19.8)%, with a mean programmed atrioventricular interval of 80 (30) ms. The mean interval between the dipyridamole SPECT thallium201 and the 123I-MIBG was 5.1 (1.7) days before the pacing period and 4.8 (1.3) days afterwards (NS).
Table 1.
Characteristics and main findings from patients and controls
| Patients (n=20) | Controls (n=20) | p Value | |
| Sex (M/F) | 10/10 | 11/9 | NS |
| Age (years) | 65.1 (6.1) | 64.9 (8.6) | NS |
| Echocardiographic findings | |||
| EDD (mm) | 47.4 (9.2) | 46.9 (8.6) | NS |
| ESD (mm) | 35.0 (7.9) | 33.9 (6.5) | NS |
| IVS (mm) | 9.8 (1.4) | 9.5 (1.3) | NS |
| PW (mm) | 9.6 (1.6) | 9.7 (0.9) | NS |
| EF (%) | 56.8 (9.9) | 57.8 (4.6) | NS |
| I123-MIBG H/M ratio | |||
| 10 min | 1.79 (0.14) | 2.07 (0.19) | <0.001 |
| 1 h | 1.74 (0.15) | 2.06 (0.16) | <0.001 |
| 4 h | 1.69 (0.17) | 2.00 (0.19) | <0.001 |
| Washout (%) | 5.46 (4.52) | 3.41 (2.22) | NS |
Values are mean (SD) or n.
EDD, end diastolic diameter; EF, left ventricular ejection fraction; ESD, end systolic diameter; F, female; H/M ratio, heart to mediastinum ratio; IVS, intraventricular septum; M, male; PW, posterior left ventricular wall; 123I-MIBG, 123I-meta-iodobenzylguanidine.
Thallium201 dipyridamole scintigraphy
In three of the 20 patients with sick sinus syndrome who were finally included in the study, thallium201 dipyridamole scintigraphy showed a reversible perfusion defect in the inferior wall which remained unchanged after the three month pacing period.
123I-MIBG scintigraphy
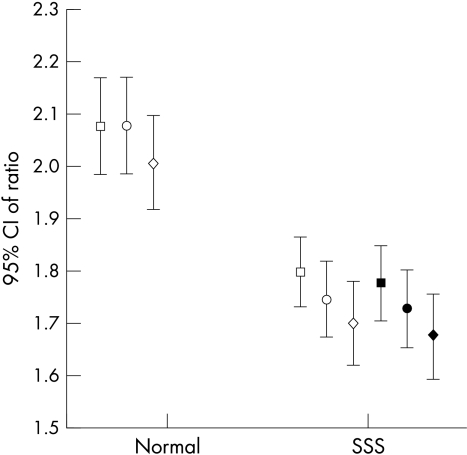
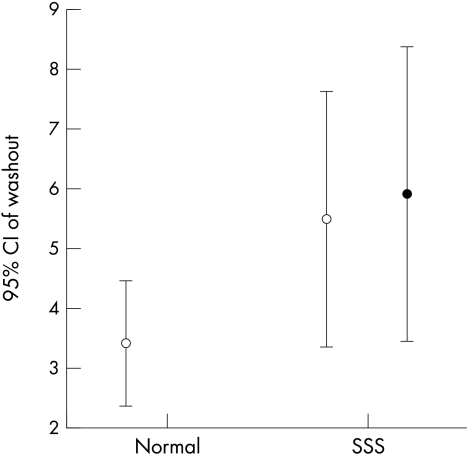
The H/M ratio was significantly lower both in early and delayed images (fig 1), while the washout rate tended to be increased in patients with sick sinus syndrome but not enough to differ significantly from the controls (fig 2). Most of the patients with sick sinus syndrome had adrenergic innervation defects in the inferior wall (16 patients) and the apical wall (12 patients); some had defects in the anterior wall (five patients) and the lateral wall (four patients), but there were fewer with defects in the posterior and septal walls (three patients each). Only one of the control group had a mild adrenergic innervation defect in the inferior wall.
Figure 1.
Time course of heart to mediastinum ratio in patients with sick sinus syndrome (SSS) and controls. Open symbols: prepacing data. Solid symbols: after three months of permanent pacing. Squares, 10 minutes; circles, 60 minutes; diamonds, 4 hours. CI, confidence interval.
Figure 2.
There was no significant difference in the washout rate between patients with sick sinus syndrome (SSS) and controls. Likewise, in the SSS group there was no significant change before and after permanent pacing. Open symbols: prepacing data. Solid symbols: after three months of permanent pacing.
The quantitative analysis showed no significant change in the H/M ratio at 10 minutes, one hour, and four hours before and after the pacemaker implantation (fig 1). Similarly, the washout rate was unchanged during the three month pacing period. Although our results did not reveal alterations in the global sympathetic activity of the left ventricular myocardium, they indicated significant regional redistribution in 123I-MIBG uptake. Specifically, aggravations of the innervation defects were noted in the inferior wall (mean defect score 1.5 (0.8) before pacing v 2.1 (0.91) after pacing; p = 0.044), in the apical wall (mean defect score 0.9 (0.9) before pacing v 1.45 (0.14) after pacing; p = 0.047), and in the posterior wall (mean defect score 0.35 (0.81) before pacing v 1.0 (1.16) after pacing; p = 0.01).
DISCUSSION
We found that patients with the sick sinus syndrome had disturbances of global and regional cardiac 123I-MIBG uptake, indicating abnormal adrenergic nerve function. We also showed that asynchronous activation induced by permanent pacing in such patients leads to regional redistribution of the myocardial 123I-MIBG uptake after a three month pacing period. These adrenergic disturbances were not associated with myocardial perfusion defects in this medium term pacing period.
Myocardial adrenergic innervation in the sick sinus syndrome group
An important finding of our study was that cardiac 123I-MIBG imaging showed significant disturbances of adrenergic innervation in the patients with sick sinus syndrome compared with the normal controls. To our knowledge only one other published report, by Matsumura and colleagues,12 has examined myocardial perfusion and innervation in the sick sinus syndrome. Those investigators suggested that impairment of the coronary microcirculation, coronary vasospasm, and cardiac sympathetic nerve dysfunction may all play a part in the pathophysiology of the syndrome. Our results also showed a significant sympathetic nervous impairment of the left ventricular myocardium, the cause of which cannot definitely be identified as anatomical or functional. The decreased H/M on delayed images may represent anatomical disturbances in nerve fibres. Though not reaching significance, washout of 123I-MIBG appeared to be increased in the patients with sick sinus syndrome compared with the controls, so we cannot exclude a contribution of functional disorders to our findings. A possible relation with the pathophysiology of bradyarrhythmic or tachyarrhythmic presentations of the syndrome needs to be explored.
Myocardial adrenergic innervation after medium term pacing period
As far as we know, this study is the first to examine the effect of permanent dual chamber pacing on myocardial perfusion and adrenergic innervation before and after pacemaker implantation in humans.
Ventricular pacing through the right ventricular apex—although the site is easily accessible and traditionally used for electrode implantation—results in asynchronous ventricular activation.13 During the last few years, “normalisation” of ventricular electrical stimulation in paced patients has acquired considerable importance. Other researchers have suggested that ventricular pacing decreases fibre shortening, contractile work, and myocardial blood flow in early activated regions and increases these indices in late activated regions.14 It is also known that long term asynchronous electrical activation leads to asymmetrical changes of left ventricular wall mass induced by regional changes of mechanical load, with the early activated regions having a lower preload than the late activated regions. Local cardiac workload regulates local cardiac mass of both myocytes and collagen, so the early activated regions become thinner while the late activated regions become thicker.15 Previous reports have also suggested that the early activated regions show functional and histological changes, reduced oxygen consumption, and disturbances of fatty acid metabolism.4,6,16
There is evidence that long term permanent right ventricular apical pacing leads to thallium201 perfusion defects,8 while it has been shown that these disturbances in regional myocardial perfusion are associated with abnormalities of myocardial blood flow,17 even in the absence of coronary artery disease.18 A recent study from our department9 examined chronically paced patients with complete heart block and revealed disturbances of the adrenergic innervation of the left ventricle, as assessed by myocardial perfusion defects shown by 123I-MIBG scintigraphy. These disturbances are described to be regional, affecting specific walls—mainly the inferior and apical—that are activated early on following right apical stimulation. However, the effect of permanent dual chamber pacing on cardiac perfusion and sympathetic activity is not entirely clear, because the studies cited above did not examine the perfusion and adrenergic innervation before pacemaker implantation. As a result the investigators cannot exclude the possibility that their findings reflect factors other than pacing. Most of the above studies examined the acute effects of right ventricular pacing or its long term results, without considering the preimplantation data.
Our results indicate that patients who are continuously paced from the right ventricular apex have a redistribution of the adrenergic innervation of the left ventricle caused by the alteration in the activation sequence. More precisely, they showed significant regional differences in early activated regions of the inferior, apical, and posterior walls after the medium term pacing period. Taking into account that global 123I-MIBG uptake does not change significantly, we postulate that our findings suggest a functional rather than an anatomical alteration of sympathetic activity. Also, given the fact that the disturbances we observed were regional in nature, it seems unlikely that they could have been caused by systemic alterations of sympathetic nerve activity. The most probable explanation is that the decline in segmental contractility in early activated regions leads to a compensatory increase in sympathetic activity in the regions in question. This results in increased competition between 123I-MIBG and noradrenaline for its uptake by the nerve terminals in the above mentioned segments, which leads to the defects in 123I-MIBG uptake.
Although regional myocardial adrenergic function has not been studied in AAI versus DDD pacing, previous investigators found accelerated global cardiac sympathetic activity in dual chamber pacing compared with atrial pacing with normal ventricular excitation,19 and increased cardiac tissue noradrenaline in paced animals,20 supporting this explanation for the abnormalities in adrenergic activation. Additionally, in a small study by Nakata and colleagues,21 more prominent 123I-MIBG scinitigraphic defects were observed in patients with VVI as compared with DDD permanent pacemakers. It remains to be determined whether the abnormalities described above imply that inhomogeneity of myocardial adrenergic innervation can lead to increased dispersion of repolarisation,22 which may be related to the high incidence of sudden cardiac death in paced patients.23,24
Although previous reports have mentioned myocardial perfusion defects on thallium201 scintigraphy in patients undergoing dual chamber pacing,8,9,18,20 we did not find deterioration in left ventricular perfusion after this medium term pacing period. However, those reports examined the long term effects of pacing on myocardial perfusion. Although it is difficult to prove, we presume that perfusion defects appear after a longer period of asynchronous ventricular activation—which is necessary before the haemodynamically compromised left ventricular function leads to histological and anatomical alterations of myocardial tissue, and in particular of the microvascular system.
Study limitations
The anatomy of the coronary vessels was not known in all our patients; for ethical reasons, coronary angiography was only undertaken in those with perfusion defects on thallium201 scintigraphy. Thus we cannot rule out the possibility that mild coronary lesions were the cause of an 123I-MIBG defect. However, the fact that our patients neither had any clinical symptoms nor any risk factors for coronary disease persuaded us that the 123I-MIBG abnormalities were not caused by coronary artery disease.
Additionally, it is possible that the higher prevalence of inferior and apical 123I-MIBG defects derived in part from artefacts occurring in these ventricular segments. However, apart from the fact that the deterioration of these defects observed in individual patients after pacing makes it unlikely that they were artefacts, our findings from patients with sick sinus syndrome were also compared with those from the controls and were found to differ significantly. Furthermore, the image quality of 123iodium is similar to that of technetium-99m and generally superior to that of thallium201,25 which is more likely to produce false positive findings as a result of attenuation of its energy in the inferior wall.
It should also be noted that we could not rule out the possibility that a proportion of the ventricular systoles during the patients' daily activity were not fully paced, even though 24 hour continuous ECG recordings showed mostly fully paced beats. As a result, we cannot be certain that the left ventricular excitation always followed the same contraction pattern. In addition, we were obliged to program the pacemakers with a short atrioventricular interval which could influence global ventricular function. However, it is very unlikely that these factors would have caused the disturbances of regional adrenergic innervation that we observed.
Conclusions
Patients with sick sinus syndrome have significant abnormalities of adrenergic innervation of the left ventricular myocardium in comparison with normal individuals. After a medium term pacing period with asynchronous ventricular electrical stimulation there is a regional redistribution of these innervation disturbances, with deterioration in the early activated regions of myocardium.
Although our findings from patients with sick sinus syndrome need further research to evaluate their clinical significance, our results derived from permanent pacing emphasise the importance of physiological synchronous ventricular electrical activation of the left ventricle, and the need for pacing techniques that ensure a normal activation–contraction sequence.
Abbreviations
H/M ratio, heart to mediastinum ratio
123I-MIBG, 123I-meta-iodobenzylguanidine
SPECT, single photon emission computed tomography
REFERENCES
- 1.Zile MR, Blaustein AS, Shimizu G, et al. Right ventricular pacing reduces the rate of left ventricular relaxation and filling. J Am Coll Cardiol 1987;10:702–9. [DOI] [PubMed] [Google Scholar]
- 2.Rosenqvist M, Bergfeldt L, Haga Y, et al. The effect of left ventricular activation sequence on cardiac performance during pacing. PACE 1996;19:1279–86. [DOI] [PubMed] [Google Scholar]
- 3.Askenazi J, Alexander JH, Koenigsberg DI, et al. Alteration of left ventricular performance by left bundle branch block simulated with atrioventricular sequential pacing. Am J Cardiol 1984;53:99–104. [DOI] [PubMed] [Google Scholar]
- 4.Adomian GE, Beazell J. Myofibrillar disarray produced in normal hearts by chronic electrical pacing. Am Heart J 1986;112:79–83. [DOI] [PubMed] [Google Scholar]
- 5.Karpawich PP, Justice CD, Chang CH, et al. Septal ventricular pacing in the immature canine heart: a new perspective. Am Heart J 1991;121:827–33. [DOI] [PubMed] [Google Scholar]
- 6.Karpawich PP, Justice CD, Cavitt DL, et al. Development sequelae of fixed-rate ventricular pacing in the immature canine heart: an electrophysiologic, hemodynamic and histopathologic evaluation. Am Heart J 1990;119:1077–83. [DOI] [PubMed] [Google Scholar]
- 7.Prinzen FW, Cheriex EC, Delhaas T, et al. Asymmetric thickness of the left ventricular wall resulting from asynchronous electrical activation: a study in dogs with ventricular pacing and in patients with left bundle branch block. Am Heart J 1995;46:489–95. [DOI] [PubMed] [Google Scholar]
- 8.Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol 1997;29:744–9. [DOI] [PubMed] [Google Scholar]
- 9.Simantirakis EN, Prassopoulos VK, Chrysostomakis SI, et al. Effects of asynchronous ventricular activation on myocardial adrenergic innervation in patients with permanent dual-chamber pacemakers. An I123-metaiodobenzylguanidine cardiac scintigraphic study. Eur Heart J 2001;22:323–32. [DOI] [PubMed] [Google Scholar]
- 10.Dae MW, O'Connell JW, Botvinick EH, et al. Scintigraphic assessment of regional cardiac adrenergic innervation. Circulation 1989;79:634–44. [DOI] [PubMed] [Google Scholar]
- 11.Merlet P, Valette H, Dubois-Rande JL, et al. Prognostic value of cardiac metaiodobenzylguanidine imaging in patients with heart failure. J Nucl Med 1992;33:471–7. [PubMed] [Google Scholar]
- 12.Matsumura K, Nakase E, Saito T, et al. Assessment of myocardial perfusion and cardiac sympathetic nerve dysfunction in patients with sick sinus syndrome – elevation of coronary hemodynamics and 201TICl/123I-MIBG myocardial SPECT. Kaku Igaku 1994;31:1321–8. [PubMed] [Google Scholar]
- 13.Lister JW, Klotz DH, Jomain SL, et al. Effect of pacemaker site on cardiac output and ventricular activation in dogs with complete heart block. Am J Cardiol 1964;14:494–503. [DOI] [PubMed] [Google Scholar]
- 14.Prinzen FW, Augustijn CH, Arts T, et al. Redistribution of myocardial fibre strain and blood flow by asynchronous electrical activation. Am J Physiol 1990;259:H300–8. [DOI] [PubMed] [Google Scholar]
- 15.Van Oosterhout MF, Prinzen FW, Arts T, et al. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 1998;98:588–95. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida H, Shirotani M, Mochizuki M, et al. Assessment of myocardial fatty acid metabolism in atrioventricular synchronous pacing: analysis of iodine 123-labeled beta-methyl iodophenyl pentadecanoic acid SPECT. J Nucl Cardiol 1999;6:33–40. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen JC, Bottcher M, Nielsen TT, et al. Regional myocardial blood flow in patients with sick sinus syndrome randomised to long-term single chamber atrial or dual chamber pacing. Effect of pacing mode and rate. J Am Coll Cardiol 2000;35:1453–61. [DOI] [PubMed] [Google Scholar]
- 18.Skalidis EI, Kochiadakis GE, Koukouraki SI, et al. Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteries. J Am Coll Cardiol 2001;37:124–9. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoga S, Nakagawa S, Fukunaga T, et al. Effect of long-term atrial-demand ventricular pacing on cardiac sympathetic activity. Nucl Med Commun 2000;21:291–7. [DOI] [PubMed] [Google Scholar]
- 20.Lee MA, Dae MW, Langberg JJ, et al. Effects of long-term right ventricular apical pacing on left ventricular perfusion, innervation, function and histology. J Am Coll Cardiol 1994;24:225–32. [DOI] [PubMed] [Google Scholar]
- 21.Nakata A, Hirota S, Tsuji H, et al. I-123 metaiodobenzylguanidine cardiac scintigraphy in patients with an implanted permanent pacemaker. Jpn Heart J 1995;36:583–91. [DOI] [PubMed] [Google Scholar]
- 22.Yoshioka K, Gao DW, Chin M, et al. Heterogeneous sympathetic innervation influences local myocardial repolarization in normally perfused rabbit hearts. Circulation 2000;101:1060–6. [DOI] [PubMed] [Google Scholar]
- 23.Mattioli AV, Rossi R, Annicchiarico E, et al. Causes of death in patients with unipolar single chamber ventricular pacing: prevalence and circumstances in dependence on arrhythmias leading to pacemaker implantation. PACE 1995;18:11–17. [DOI] [PubMed] [Google Scholar]
- 24.Zehender M, Buchner C, Meinertz T, et al. Prevalence, circumstances, mechanisms, and risk stratification of sudden death in unipolar single-chamber ventricular pacing. Circulation 1992;85:596–605. [DOI] [PubMed] [Google Scholar]
- 25.Gullon SJ. Principles of cardiac SPECT. In: De Puey GE, Berman DS, Garcia EV, eds. Cardiac SPECT imaging. New York: Raven Press, 1995:3.