Abstract
Objective: To compare the early and late outcomes of primary percutaneous transluminal coronary angioplasty (PTCA) with fibrinolytic treatment among diabetic patients with acute myocardial infarction (AMI).
Design: Retrospective observational study with data obtained from prospective registries.
Setting: Tertiary cardiovascular institution with 24 hour acute interventional facilities.
Patients: 202 consecutive diabetic patients with AMI receiving reperfusion treatment within six hours of symptom onset.
Interventions: Fibrinolytic treatment was administered to 99 patients, and 103 patients underwent primary PTCA. Most patients undergoing PTCA received adjunctive stenting (94.2%) and glycoprotein IIb/IIIa inhibition (63.1%).
Main outcome measures: Death, non-fatal reinfarction, and target vessel revascularisation at 30 days and one year were assessed.
Results: Baseline characteristics were similar in these two treatment groups except that the proportion of patients with Killip class III or IV was considerably higher in those treated with PTCA (15.5% v 6.1%, p = 0.03) and time to treatment was significantly longer (103.7 v 68.0 minutes, p < 0.001). Among those treated with PTCA, the rates for in-hospital recurrent ischaemia (5.8% v 17.2%, p = 0.011) and target vessel revascularisation at one year (19.4% v 36.4%, p = 0.007) were lower. Death or reinfarction at one year was also reduced among those treated with PTCA (17.5% v 31.3%, p = 0.02), with an adjusted relative risk of 0.29 (95% confidence interval 0.15 to 0.57) compared with fibrinolysis.
Conclusion: Among diabetic patients with AMI, primary PTCA was associated with reduced early and late adverse events compared with fibrinolytic treatment.
Keywords: angioplasty, diabetes, fibrinolysis, myocardial infarction
Even with the widespread availability of fibrinolytic treatment, diabetes mellitus remains an important adverse prognostic factor in patients with acute myocardial infarction (AMI).1–3 Although recent evidence from randomised trials has shown that primary percutaneous transluminal coronary angioplasty (PTCA) provides significantly better clinical outcomes than fibrinolysis for AMI in general,4–9 its effect on diabetic patients remains unclear. A post hoc subgroup analysis of the GUSTO-IIb (global use of strategies to open occluded coronary arteries in acute coronary syndromes) angioplasty substudy found modest improvements in short and long term outcomes in diabetic patients treated with primary PTCA compared with fibrinolysis.10 Whether these favourable results are broadly applicable to non-selected diabetic patients with AMI is unknown. Accordingly, the objective of this study was to evaluate the early and late outcomes of primary PTCA compared with fibrinolytic treatment for AMI among diabetic patients in current practice.
METHODS
Patient population
During the period from January 1997 to December 1999, a total of 651 patients presenting directly to our institution with ST segment elevation AMI received reperfusion treatment within six hours of symptom onset. Patients eligible for the study were identified from prospective registries. Diabetes mellitus was considered present if the patient had been informed of the diagnosis or was on prescribed treatment. There were 202 (31%) diabetic patients, of whom 99 received fibrinolytic treatment and 103 underwent primary PTCA. Following standard institutional practice, all eligible patients were routinely offered pharmacological or mechanical reperfusion treatments and the potential risks and benefits were carefully explained to them. The patient made the final decision regarding the mode of reperfusion treatment. Informed consent was obtained from all patients.
Fibrinolytic treatment
Seventy nine patients (80%) received streptokinase (1.5 MU intravenously over one hour). The accelerated recombinant tissue-type plasminogen activator (rt-PA) regimen (15 mg intravenous bolus followed by an infusion of 50 mg over 30 minutes, then 35 mg over the next hour) was administered to 10 patients. The remaining 10 patients received a reduced dose rt-PA regimen (15 mg intravenous bolus followed by 35–50 mg in one hour) plus a bolus (0.25 mg/kg) and 12 hour infusion (0.125 μg/kg/min, to a maximum of 10 μg/min) of abciximab as part of a research protocol. All patients treated with rt-PA also received intravenous heparin (5000 U bolus followed by an infusion titrated to maintain an activated partial thromboplastin time of 60–80 seconds). Successful fibrinolysis was defined clinically as complete resolution of chest pain together with ≥ 50% resolution of the ST segment elevation in the worst lead.11, 12 The time from arrival at the emergency department to the start of administration of a fibrinolytic agent was recorded as the time to treatment.
Primary PTCA
Primary angioplasty was performed in 103 patients using standard techniques with the aim of re-establishing antegrade flow in the infarct related artery as soon as possible. Only the infarct related artery was treated. Successful angioplasty was defined as post-treatment residual stenosis < 30% with TIMI (thrombolysis in myocardial infarction) flow grade 3. Heparin was administered during the procedure to keep the activated clotting time ≥ 250 seconds. Almost all the patients (94.2%) received adjunctive coronary stenting and a glycoprotein IIb/IIIa inhibitor was administered to 65 of them (63.1%). The time of arrival at the emergency department to the time of first balloon inflation was recorded as time to treatment.
Adjunctive medical treatment
All patients received aspirin (300 mg at presentation followed by 100 mg daily), while other medications were administered at the discretion of the attending cardiologist. In addition, all patients who underwent primary angioplasty were treated with a second antiplatelet agent (either ticlopidine 250 mg twice daily or clopidogrel 75 mg daily, after an initial loading dose) during the procedure and for at least four weeks.
Data collection
The clinical data on primary PTCA were obtained from a prospective registry and from review of the patients’ clinical records. Baseline demographic, clinical, and angiographic data were collected. Patients who received fibrinolytic treatment were identified from our coronary care unit registry and data were collected retrospectively. Information regarding follow up visits, subsequent hospitalisation, and adverse cardiac events was obtained from hospital charts, telephone interviews, and the national death registry.
End points
The main end points of our analysis were all cause mortality and a composite of death and non-fatal reinfarction during the index hospitalisation, at 30 days and one year. Reinfarction was defined as recurrence of chest pain lasting ≥ 20 minutes with either new ST segment elevation on the ECG or repeat increase of cardiac enzyme levels. Other variables assessed were recurrent ischaemia (recurrent chest pain lasting ≥ 10 minutes with new ST segment changes on the ECG but no repeat increase of cardiac enzyme levels), congestive cardiac failure, and target vessel revascularisation (TVR) during the index hospitalisation, as well as subsequent readmissions for angina, congestive cardiac failure, and TVR at 30 days and one year. Bleeding complications were defined based on clinical assessment. It was defined as severe if intracranial haemorrhage or bleeding causing haemodynamic compromise had occurred and moderate if blood transfusion was required without the occurrence of haemodynamic compromise. Bleeding without haemodynamic effects and not requiring transfusion was considered minor.
Statistical analysis
We used SPSS version 9.0 (SPSS Inc, Chicago, Illinois, USA) for data management and statistical analysis. Continuous variables are expressed as mean (SD) and differences between the two groups of patients were tested using Student’s t test or analysis of variance. Categorical variables are expressed as numbers and percentages and the groups were compared using likelihood ratio χ2 or Fisher’s exact tests. Event-free survival at one year was determined for each reperfusion strategy by the Kaplan-Meier method and homogeneity of the survival curves was tested with the use of both the log rank test and the Wilcoxon rank sum test.
Cox proportional hazards analysis was used to assess the relation between reperfusion strategies for the occurrence of death and death or reinfarction at one year. A multivariate model was constructed to examine the individual and joint relation between the baseline clinical characteristics (table 1) and the clinical outcome. Candidate predictor variables that were identified as important predictors of the clinical outcome by univariate analysis (p < 0.10) were included in the final multivariate Cox model. The relation between the variables and outcome is reported as the relative risk with 95% confidence intervals (CI). All tests of significance were two tailed. Differences between groups were considered significant at p < 0.05.
Table 1.
Baseline demographic and clinical characteristics
| Fibrinolysis (n = 99) | Angioplasty (n = 103) | p Value | |
| Demographics | |||
| Age (years (SD)) | 60.7 (10.1) | 59.5 (9.2) | 0.40 |
| Age ≥ 65 years | 38 (38.4%) | 35 (34.0%) | 0.52 |
| Male sex | 73 (73.7%) | 72 (69.9%) | 0.55 |
| Chinese ethnicity | 53 (53.5%) | 66 (64.1%) | NS |
| Insulin treated | 12 (12.1%) | 11 (10.7%) | 0.75 |
| Risk factors | |||
| Hypertension | 58 (58.6%) | 65 (63.1%) | 0.51 |
| Hypercholesterolaemia | 93 (93.9%) | 93 (90.3%) | 0.34 |
| Smoking (current) | 52 (52.5%) | 43 (41.7%) | 0.13 |
| Family history of coronary disease | 21 (21.2%) | 14 (13.6%) | 0.15 |
| Cardiac history | |||
| MI | 15 (15.2%) | 14 (13.6%) | 0.75 |
| Coronary angioplasty | 1 (1.0%) | 5 (4.9%) | 0.21 |
| Bypass surgery | 2 (2.0%) | 2 (1.9%) | 1.0 |
| Heart failure | 3 (3.0%) | 4 (3.9%) | 1.0 |
| Non-cardiac history | |||
| Stroke | 4 (4.0%) | 7 (6.8%) | 0.54 |
| Renal impairment | 3 (3.0%) | 8 (7.8%) | 0.26 |
| Clinical presentation | |||
| Time from onset to arrival (hours (SD)) | 3.6 (2.5) | 3.5 (2.6) | 0.72 |
| Time from arrival to therapy (minutes (SD)) | 68.0 (31.3) | 103.7 (40.0) | <0.001 |
| Killip class | |||
| I | 68 (68.7%) | 62 (60.2%) | 0.21 |
| II | 25 (25.3%) | 25 (24.3%) | 0.87 |
| III | 5 (5.1%) | 5 (4.9%) | 1.00 |
| IV | 1 (1.0%) | 11 (10.7%) | 0.005 |
| ≥III | 6 (6.1%) | 16 (15.5%) | 0.03 |
| Average Killip class | 1.38 (0.63) | 1.66 (0.99) | 0.019 |
| Electrocardiographic features | |||
| Anterior MI | 43 (43.4%) | 53 (51.5%) | 0.25 |
| Inferior MI with RV involvement | 16 (16.2%) | 15 (14.6%) | 0.75 |
| Inferior MI (precordial ST depression) | 26 (26.3%) | 27 (26.3%) | 0.99 |
| Inferior MI (isolated) | 14 (14.1%) | 8 (7.8%) | 0.15 |
MI, myocardial infarction; RV, right ventricle.
RESULTS
Baseline characteristics
The baseline demographic and clinical characteristics of the patients were comparable between these two treatment groups (table 1). However, the proportion of patients in Killip class III or IV was greater among those treated with primary PTCA (15.5% v 6.1%, p = 0.03). The mean time to treatment was also significantly longer for patients receiving primary PTCA than for those receiving fibrinolytic treatment (104 v 68 minutes, p < 0.001).
Angiographic data
Among patients treated with fibrinolysis, 66 (67%) underwent coronary angiography during the index hospitalisation period. The remaining patients declined to undergo the procedure. There was no significant difference in the location of the infarct related vessel and extent of coronary artery disease between these two groups of patients (table 2).
Table 2.
Angiographic data
| Fibrinolysis (n = 66) | Angioplasty (n = 103) | p Value | |
| Extent of coronary artery disease | |||
| Single vessel | 16 (24.2%) | 25 (24.3%) | 0.75 |
| Double vessel | 27 (40.9%) | 42 (40.8%) | 0.99 |
| Triple vessel | 23 (34.8%) | 36 (34.9%) | 0.99 |
| Infarct related artery | |||
| Left anterior descending | 24 (36.3%) | 52 (50.5%) | 0.07 |
| Left circumflex | 10 (15.2%) | 8 (7.8%) | 0.13 |
| Right coronary | 31 (47.0%) | 41 (39.8%) | 0.36 |
| Other (grafts) | 1 (1.5%) | 2 (1.9%) | 1.00 |
In-hospital outcomes
Treatment failure was considerably higher among patients receiving fibrinolysis (12.1% v 2.9%, p = 0.02). In addition, primary PTCA was associated with a lower occurrence of recurrent ischaemia (5.8% v 17.2%, p = 0.011) and TVR procedures (5.8% v 19.2%, p = 0.004) as shown in table 3. There were no significant differences in the incidence of major bleeding complications and duration of hospitalisation. Two cases of ischaemic stroke occurred in each group and one case of haemorrhagic stroke on the first day in the fibrinolytic group. No significant difference was observed between the two groups.
Table 3.
In-hospital events
| Fibrinolysis (n = 99) | Angioplasty (n = 103) | p Value | |
| Primary end points | |||
| Death | 9 (9.1%) | 7 (6.8%) | 0.55 |
| Reinfarction | 1 (1.0%) | 0 | 0.49 |
| Composite (death + reinfarction) | 10 (10.1%) | 7 (6.8%) | 0.39 |
| Other cardiac events | |||
| Recurrent ischaemia | 17 (17.2%) | 6 (5.8%) | 0.011 |
| Heart failure | 13 (13.1%) | 17 (16.5%) | 0.50 |
| Inhospital revascularisation | 19 (19.2%) | 6 (5.8%) | 0.004 |
| Non-cardiac events | |||
| Bleeding (overall) | 15 (15.2%) | 10 (9.7%) | 0.24 |
| Bleeding (moderate and severe) | 4 (4.0%) | 5 (4.9%) | 1.00 |
| Stroke (haemorrhagic) | 1 (1.0%) | 0 | 0.49 |
| Stroke (ischaemic) | 2 (2.0%) | 2 (1.9%) | 1.00 |
| Duration of hospitalisation (days (SD)) | 8.21 (4.98) | 7.65 (3.64) | 0.36 |
Thirty day outcomes
Readmission for angina (2.9% v 11.1%, p = 0.027) and TVR (10.7% v 27.3%, p = 0.003) were significantly lower among patients treated with PTCA (table 4). However, there was no significant difference in the incidence of death or non-fatal reinfarction.
Table 4.
Events at 30 days and one year of follow up
| Fibrinolysis (n=99) | Angioplasty (n=103) | p Value | |
| Primary end points at 30 days | |||
| Death | 12 (12.1%) | 8 (7.8%) | 0.30 |
| Reinfarction | 2 (2.0%) | 3 (2.9%) | 1.00 |
| Composite (death + reinfarction) | 14 (14.1%) | 11 (10.7%) | 0.46 |
| Other cardiac events at 30 days | |||
| Angina | 11 (11.1%) | 3 (2.9%) | 0.027 |
| Heart failure | 14 (14.1%) | 19 (18.4%) | 0.41 |
| Target vessel revascularisation | 27 (27.3%) | 11 (10.7%) | 0.003 |
| Primary end points at one year | |||
| Death | 18 (18.2%) | 10 (9.7%) | 0.08 |
| Reinfarction | 13 (13.1%) | 8 (7.8%) | 0.21 |
| Composite (death + reinfarction) | 31 (31.3%) | 18 (17.5%) | 0.022 |
| Other cardiac events at one year | |||
| Angina | 24 (24.2%) | 20 (19.4%) | 0.41 |
| Heart failure | 17 (17.2%) | 22 (21.4%) | 0.45 |
| Target vessel revascularisation | 36 (36.4%) | 20 (19.4%) | 0.007 |
One year outcomes
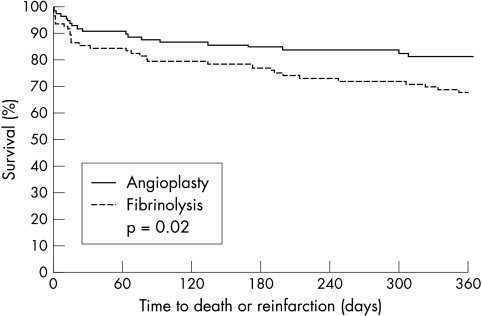
Primary PTCA was associated with a trend towards a lower incidence of death at one year (9.7% v 18.2%, p = 0.08). Death and non-fatal reinfarction were significantly less frequent in the PTCA group than in the fibrinolysis group (17.5% v 31.3%, p = 0.022), as fig 1 shows. The frequency of TVR procedures remained significantly lower among patients treated with PTCA (19.4% v 36.4%, p = 0.007). By one year, there was little difference in the rates of readmission for angina and heart failure between the two groups of patients.
Figure 1.
Kaplan-Meier curves depicting survival free of death or reinfarction for diabetic patients treated with fibrinolysis or angioplasty at 12 months of follow up.
Multivariate model
In our multivariate model, primary PTCA was associated with a significantly lower risk of mortality or reinfarction at one year (table 5). Older patients (age ≥ 65 years), those in a higher Killip class, and insulin treatment conferred a higher risk. Patients with an inferior myocardial infarction associated with precordial ST segment depression also had a greater likelihood of death or reinfarction at one year.
Table 5.
Correlates of death and composite end point (death + non-fatal reinfarction) at one year by multivariate analysis
| Variable | RR | 95% CI | p Value |
| Death at one year | |||
| Treated with primary angioplasty | 0.20 | 0.08 to 0.53 | 0.001 |
| Age ≥ 65 years | 2.22 | 0.99 to 4.99 | 0.05 |
| Insulin treated diabetes | 4.13 | 1.23 to 13.85 | 0.02 |
| Killip class ≥III | 10.30 | 4.19 to 25.29 | <0.001 |
| Death + non-fatal reinfarction at one year | |||
| Treated with primary angioplasty | 0.29 | 0.15 to -0.57 | <0.001 |
| Age ≥ 65 years | 2.04 | 1.12 to 3.72 | 0.02 |
| Insulin treated diabetes | 2.56 | 1.22 to 5.38 | 0.01 |
| Inferior MI (precordial ST depression) | 2.81 | 1.51 to 5.23 | 0.01 |
| Killip class ≥III | 5.08 | 2.51 to 10.29 | <0.001 |
CI, confidence interval; RR, relative risk
DISCUSSION
Among patients with diabetes and AMI, our study showed that primary PTCA was associated with lower rates of recurrent ischaemia and TVR procedures and a trend towards a reduction in all cause mortality at one year compared with fibrinolysis. This benefit was achieved without an increase in bleeding complications. Importantly, the combined incidence of death and non-fatal reinfarction at one year was substantially lower among those treated with primary PTCA. These results corroborated the observations in a previous small study of 32 diabetic patients with AMI,13 in which primary PTCA was found to be associated with a lower long term mortality than fibrinolysis (adjusted relative risk of mortality was 3.2 times higher for patients treated with fibrinolysis).
Improved techniques and operator experience have established primary PTCA as an excellent alternative reperfusion treatment for AMI. Although its impact on diabetic patients with AMI was less certain, there has been encouraging evidence to support its use.14, 15 The results of primary PTCA have been further improved by coronary stenting, both in the general population16–19 and in diabetic patients.20 However, stent thrombosis and major cardiovascular events still occurred more frequently. A recent study of primary stenting for AMI in diabetic compared with non-diabetic patients found a much higher incidence of stent thrombosis in diabetic patients (18% v 1%, p = 0.003), as well as a higher risk of one month (relative risk 9.89, 95% CI 1.6 to 30) and late (relative risk 8.39, 95% CI 2.93 to 24) major cardiovascular events.20
The administration of potent antiplatelet agents, such as the glycoprotein IIb/IIIa receptor inhibitors, during elective PTCA has been shown to improve outcome. In the EPILOG (evaluation of PTCA to improve long-term outcome by c73E3 glycoprotein IIb/IIIa receptor blockade) study,21 the use of abciximab was associated with a decrease in the occurrence of death or myocardial infarction in diabetic patients undergoing PTCA. Similarly, a post hoc subgroup analysis from the EPISTENT (evaluation of platelet IIb/IIIa inhibitor for stenting trial) showed a complementary benefit when abciximab was administered during coronary stenting for patients with diabetes.22, 23 Conversely, a recent analysis on unselected patients showed that abciximab did not prevent the occurrence of late adverse events in diabetic patients undergoing elective PTCA and coronary stenting.24 However, the patients in this study who received abciximab had poorer left ventricular function and more extensive coronary artery disease. In the context of AMI, the preliminary results of the CADILLAC (controlled abciximab and device investigation to lower late angioplasty complications) study suggested that, among patients undergoing direct PTCA without stenting, abciximab use reduced mortality (4.3% v 2.3%) and enhanced event-free survival at six months (19.3% v 15.2%). No mortality benefit was observed with abciximab if coronary stenting was performed. However, these findings are derived from a non-randomised population. Hence, the role of glycoprotein IIb/IIIa inhibition during direct PTCA with adjunctive stenting in diabetic patients remains unclear.
The GUSTO IIb angioplasty substudy showed a significant reduction in recurrent ischaemia and reinfarction in diabetic patients treated with primary PTCA compared with fibrinolysis.10 However, there was no survival benefit with PTCA. In fact, mortality appeared to be higher in patients treated with PTCA at six months, although at 12 months survival was similar for the two treatment modalities. Of note, coronary stenting and glycoprotein IIb/IIIa receptor inhibitors were rarely used in this study while, in contrast, our study patients had a high rate of adjunctive stenting (94%) and glycoprotein IIb/IIIa inhibition (63%). These factors could have contributed favourably to our results.25 More recently, the routine use of clopidogrel in patients with unstable angina has been shown to improve outcome.26 While patients after stenting were treated with a thienopyridine, which could theoretically have contributed to an improvement in outcome, data on its use in ST elevation AMI are lacking.
In our study, the significant adverse prognostic factors for diabetic patients with AMI were not different from previous studies. Insulin treated diabetes, advanced age, a higher Killip class (III or IV), and inferior myocardial infarction with concomitant precordial ST segment depression have all been shown to adversely affect outcome.2, 4, 27, 28 However, other common predictors of adverse outcome, especially anterior infarction and a previous history of myocardial infarction, cardiac failure, or cerebrovascular events,3, 8, 9 were not found in our study.
The better results for primary PTCA may be the result of several possible mechanisms. For AMI, early outcome depends on establishing complete reperfusion as quickly as possible and this can be achieved effectively by PTCA compared with fibrinolysis, even though the mean time to treatment for PTCA is usually longer.5, 7 As shown in previous studies, diabetic patients may have a greater residual lesion in the infarct related artery after treatment with fibrinolytics, resulting in a higher rate of recurrent ischaemia.29 The higher risk of adverse events may be caused by enhanced thrombogenicity and impaired fibrinolysis.30, 31 Furthermore, glycoprotein IIb/IIIa inhibition during primary PTCA has been shown to improve microvascular flow,32 which may be particularly important for diabetic patients. However, the long term outcome of these patients depends on the extent of coronary disease and residual left ventricular function, as well as the presence of other risk factors.25 Hence, aggressive secondary preventive measures such as tight glycaemic control and lipid lowering may be just as important as the mode of reperfusion treatment for these patients.
Study limitations
This is not a randomised study and thus has its inherent limitations. The higher proportion of patients with pulmonary oedema and cardiogenic shock among those treated with primary PTCA may have confounded our results, although they are probably a more accurate reflection of common clinical practice at this time. Nonetheless, the sicker patients in the PTCA group may have reduced the degree of benefit seen in this study. Another limitation was that the majority of our patients in the fibrinolytic group (80%) were treated with streptokinase instead of rt-PA. Previous treatment trials have shown that treatment with rt-PA resulted in improved survival at 30 days and one year,33, 34 with a relative risk reduction of 14% and 10%, respectively. As such, the outcome among our patients treated with fibrinolytic agents may have been better if rt-PA had been used more frequently, although the absolute difference would likely have been small. Streptokinase is still the most commonly used fibrinolytic agent in many countries and hence our findings are still largely applicable. The relatively small sample size is also a limitation, though to our knowledge this is the largest for such a study. Another potential limitation is that the follow up period for this study is not sufficiently long enough to address the issue of long term outcome in such patients.
Conclusion
In diabetic patients with AMI, primary angioplasty, especially with adjunctive coronary stenting and glycoprotein IIb/IIIa inhibition, was associated with lower rates of early and late adverse events than with fibrinolytic treatment. Hence, as in patients without diabetes, PTCA is an attractive alternative to fibrinolytic treatment if the necessary facilities and trained personnel are available.
Abbreviations
AMI, acute myocardial infarction
CADILLAC, controlled abciximab and device investigation to lower late angioplasty complications
EPILOG, evaluation of PTCA to improve long-term outcome by c73E3 glycoprotein IIb/IIIa receptor blockade
EPISTENT, evaluation of platelet IIb/IIIa inhibitor for stenting trial
GUSTO-IIb, global use of strategies to open occluded coronary arteries in acute coronary syndromes
PTCA, percutaneous transluminal coronary angioplasty
rt-PA, recombinant tissue-type plasminogen activator
TIMI, thrombolysis in myocardial infarction
TVR, target vessel revascularisation
REFERENCES
- 1.Woodfield SL, Lundergan CF, Reiner JS, et al. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol 1996;28:1661–9. [DOI] [PubMed] [Google Scholar]
- 2.Mak KH, Moliterno DJ, Granger CB, et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction. J Am Coll Cardiol 1997;30:171–9. [DOI] [PubMed] [Google Scholar]
- 3.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. Circulation 1995;91:1659–68. [DOI] [PubMed] [Google Scholar]
- 4.Grines CL, Browne KF, Marco J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1993;328:673–9. [DOI] [PubMed] [Google Scholar]
- 5.Zijlstra F, de Boer MJ, Hoorntje JCA, et al. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med 1993;328:680–4. [DOI] [PubMed] [Google Scholar]
- 6.The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes GUSTO IIb Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med 1997;336:1621–8. [DOI] [PubMed] [Google Scholar]
- 7.Weaver DW, Simes RJ, Betriu A, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction. A quantitative review. JAMA 1997;278:2093–8. [PubMed] [Google Scholar]
- 8.Nunn CM, O’Neill WW, Rothbaum D, et al. Long-term outcome after primary angioplasty: report from the primary angioplasty in myocardial infarction (PAMI-I) trial. J Am Coll Cardiol 1999;33:640–6. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra F, Hoorntje JCA, de Boer MJ, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1999;341:1413–9. [DOI] [PubMed] [Google Scholar]
- 10.Hasdai D, Granger CB, Srivatsa SS, et al. Diabetes mellitus and outcome after primary coronary angioplasty for acute myocardial infarction: Lessons from the GUSTO-IIb angioplasty substudy. J Am Coll Cardiol 2000;35:1502–12. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez AR, Sequeira RF, Chakko S, et al. ST segment tracking for rapid determination of patency of the infarct-related artery in acute myocardial infarction. J Am Coll Cardiol 1995;26:675–83. [DOI] [PubMed] [Google Scholar]
- 12.Langer A, Krucoff MW, Klootwijk P, et al. Noninvasive assessment of speed and stability of infarct-related artery reperfusion: results of the GUSTO ST segment monitoring study. J Am Coll Cardiol 1995;25:1552–7. [DOI] [PubMed] [Google Scholar]
- 13.Thomas K, Ottervanger JP, de Boer MJ, et al. Primary angioplasty compared with thrombolysis in acute myocardial infarction in diabetic patients. Diabetes Care 1999;22:647–8. [DOI] [PubMed] [Google Scholar]
- 14.Waldecker B, Waas W, Haberbosch W, et al. Type 2 diabetes and acute myocardial infarction. Angiographic findings and results of an invasive therapeutic approach in type 2 diabetic versus nondiabetic patients. Diabetes Care 1999;22:1832–8. [DOI] [PubMed] [Google Scholar]
- 15.Mak KH, Topol EJ. Emerging concepts in the management of acute myocardial infarction in patients with diabetes mellitus. J Am Coll Cardiol 2000;35:563–8. [DOI] [PubMed] [Google Scholar]
- 16.Antoniucci D, Santoro GM, Bolognese L, et al. A clinical trial comparing primary stenting of the infarct-related artery with optimal primary angioplasty for acute myocardial infarction. Results from the Florence randomized elective stenting in acute coronary occlusion (FRESCO) trial. J Am Coll Cardiol 1998;31:1234–9. [DOI] [PubMed] [Google Scholar]
- 17.Suryapranata H, van’t Hof AWJ, Hoorntje JCA, et al. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation 1998;97:2502–5. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Brodie BR, Griffin JJ, et al. Prospective, multicenter study of the safety and feasibility of primary stenting in acute myocardial infarction: in-hospital and 30-day results of the PAMI stent pilot trial. Primary angioplasty in myocardial infarction stent pilot trial investigators. J Am Coll Cardiol 1998;31:23–30. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Brodie BR, Griffin JJ, et al. Clinical and angiographic follow-up after primary stenting in acute myocardial infarction: the primary angioplasty in myocardial infarction (PAMI) stent pilot trial. Circulation 1999;99:1548–54. [DOI] [PubMed] [Google Scholar]
- 20.Silva JA, Ramee SR, White CJ, et al. Primary stenting in acute myocardial infarction: influence of diabetes mellitus in angiographic results and clinical outcome. Am Heart J 1999;138:446–55. [DOI] [PubMed] [Google Scholar]
- 21.Kleiman NS, Lincoff AM, Kereiakes DJ, et al. Diabetes mellitus, glycoprotein IIb/IIIa blockade, and heparin: evidence for a complex interaction in a multicenter trial. Circulation 1998;97:1912–20. [DOI] [PubMed] [Google Scholar]
- 22.Lincoff AM, Califf RM, Moliterno DJ, et al. Complementary clinical benefits of coronary artery stenting and blockade of platelet glycoprotein IIb/IIIa receptors. Evaluation of platelet IIb/IIIa Inhibition in stenting investigators. N Engl J Med 1999;341:319–27. [DOI] [PubMed] [Google Scholar]
- 23.Marso SP, Lincoff AM, Ellis SG, et al. Optimizing the percutaneous interventional outcomes for patients with diabetes mellitus. Results of the EPISTENT (evaluation of platelet IIb/IIIa inhibition in stenting trial) diabetic substudy. Circulation 1999;100:2477–84. [DOI] [PubMed] [Google Scholar]
- 24.Velianou JL, Verghese M, Wilson SH, et al. Effect of abciximab on late adverse events in patients with diabetes mellitus undergoing stent implantation. Am J Cardiol 2000;86:1063–8. [DOI] [PubMed] [Google Scholar]
- 25.King SB III. Acute myocardial infarction: are diabetics different? J Am Coll Cardiol 2000;35:1513–5. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Zhao F, Mehta SR, et al; The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 27.Brodie BR, Grines CL, Ivanhoe R, et al. Six-month clinical and angiographic follow-up after direct angioplasty for acute myocardial infarction: final results from the primary angioplasty registry. Circulation 1994;25:156–62. [DOI] [PubMed] [Google Scholar]
- 28.Ribichini F, Steffenino G, Dellavalle A, et al. Comparison of thrombolytic therapy and primary coronary angioplasty with liberal stenting for inferior myocardial infarction with precordial ST-segment depression. Immediate and long-term results of a randomized study. J Am Coll Cardiol 1998;32:1687–94. [DOI] [PubMed] [Google Scholar]
- 29.Gray RP, Yudkin JS, Patterson DL. Enzymatic evidence of impaired reperfusion in diabetic patients after thrombolytic therapy for acute myocardial infarction. Br Heart J 1993;70:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGill JB, Schneider DJ, Arfken CL, et al. Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes 1994;43:104–9. [DOI] [PubMed] [Google Scholar]
- 31.Piemontino U, Ceriello A, di Minno G. Hemostatic and metabolic abnormalities in diabetes mellitus. The search for a link. Haematologica 1994;79:387–92. [PubMed] [Google Scholar]
- 32.Agati L, Voci P, Hickle P, et al. Tissue-type plasminogen activator therapy versus primary coronary angioplasty: impact on myocardial tissue perfusion and regional function 1 month after uncomplicated myocardial infarction. J Am Coll Cardiol 1998;31:338–43. [DOI] [PubMed] [Google Scholar]
- 33.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. [DOI] [PubMed] [Google Scholar]
- 34.Califf RM, White HD, van de Werf F, et al. One-year results from the global utilization of streptokinase and TPA for occluded coronary arteries (GUSTO-I) trial. GUSTO investigators. Circulation 1996:94:1233–8. [DOI] [PubMed] [Google Scholar]