Recent work suggests that myocardial hypoxia or ischaemia are also pathophysiologic factors in idiopathic dilated cardiomyopathy (IDC).1 Besides several other factors (increased wall stress, endothelial dysfunction, decreased coronary reserve), the observed decreased capillarisation in IDC, disproportionate to the rate of hypertrophy, may further contribute to this oxygen demand–supply mismatch. The reason for this seemingly decreased angiogenic capacity remains unclear, however a role for microvascular abnormalities in heart failure is now recognised.2
Hypoxia is the key factor in the induction of vascular endothelial growth factor (VEGF). Increased expression of VEGF causes angiogenesis, and expression level of VEGF could therefore mediate the capillarisation in IDC. Recently, data have been reported on this issue,3 and the authors found a downregulation of VEGF165 and VEGF189 isoforms. The VEGF121 however was not investigated, although it has been suggested that this isoform in particular has powerful angiogenic capacities. Additionally, it is unclear whether VEGF expression is related to the severity of the disease.
SUBJECTS AND METHODS
We analysed 28 patients with IDC. Patients had enlarged left ventricular end diastolic and systolic diameters (LVEDD 69 (2.1) mm, LVESD 61 (2.6) mm), and decreased left ventricular ejection fraction (LVEF) (0.27 (0.03)), and elevated wedge (15 (1.9) mm Hg) and left ventricular end diastolic pressures (14.1 (2.7) mm Hg). Echocardiography did not reveal cardiac disease in the 10 brain dead subjects, who served as controls. Endomyocardial biopsy taken from the right ventricle showed cardiomyocyte hypertrophy and interstitial fibrosis. Biopsies were snap frozen and stored at −80°C. Total RNA was isolated using the acid guanidium thiocyanate lysis method, as described before.4 First strand cDNA was synthesised from 1 μg RNA using the RT-PCR Core kit (Perkin Elmer, USA). The cDNA of interest and of the housekeeping enzyme glyceraldehyde-3-dehydrogenase (GAPDH) were co-amplified in a semiquantitative PCR. The VEGF primers5 span the splice junctions allowing separation by electrophoresis; bands were evaluated by densitometry. To validate the PCRs, the changes in ratios for VEGF121, VEGF165, and VEGF189 were determined and correlated to the amount of input template. PCR products were ligated into pGEM-T-easy-vector and transformed in JM109 cells (pGEM-T-easy kit, Promega, Netherlands). Cell cultures were grown and increasing amounts of the gene of interest were added to a fixed amount of GAPDH in a PCR mixture, containing both GAPDH and VEGF primer. The ratios of VEGF versus GAPDH were calculated, and related to absolute amounts of input template. For Western blot analysis, whole cell protein extracts were obtained from left ventricular tissue and assayed, as described.4 Membranes were incubated with primary antibodies: polyclonal anti-rabbit VEGF antibody (SC-152; Santa Cruz Biotechnology, Netherlands) and monoclonal anti-rabbit GAPDH (Affinity Bioreagents, Golden, Colorado, USA), and signals were detected by GARpo (Santa Cruz, Sanvertech BV, Heerhugowaard, Netherlands) with ECL detection (Amersham, Roosendaal, Netherlands).
Data are reported as means (SEM). For comparisons between groups an unpaired student t test, χ2, and a Wilcoxon two sample test were used. Assays were performed three times; p < 0.05 was considered significant.
RESULTS
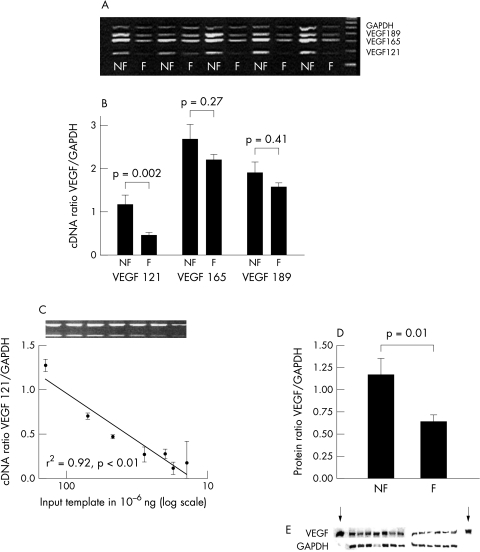
The expression of VEGF121, VEGF165, and VEGF189 isoforms (PCR products of 177 bp, 312 bp, and 384 bp on the gel, respectively) is displayed in fig 1A. GAPDH was expressed at similar levels in controls (non-failing or NF) and in IDC patients (failing or F). Figure 1B shows that cDNA ratio of VEGF121/GAPDH is significantly reduced in failing myocardium as compared to controls (0.47 (0.04) v 1.2 (0.21) = 40%; p = 0.002). VEGF165 (2.2 (0.13) v 2.7 (0.24) = 82%; p = 0.27) and VEGF189 (1.6 (0.09) v 1.89 (0.24) = 83%; p = 0.41) exhibit a similar trend. The amount of input template strongly correlated (p < 0.01) with the observed signals for all isoforms (fig 1C: VEGF121; VEGF165: r2 = 0.98; VEGF189: r2 = 0.99). Protein VEGF/GAPDH ratios (fig 1D,E) are decreased in IDC patients compared to controls (1.17 (0.18) v 0.64 (0.08) = 55%; p = 0.01). No correlations between clinical parameters and VEGF expression levels were established.
Figure 1.
(A) Ethidium bromide stained 1.5% agarose gel of expression of mRNA of VEGF and GAPDH in heart tissue from patients (failing or F) and controls (non-failing or NF). (B) Plot showing the mean cDNA ratios for VEGF121, VEGF165, and VEGF189, normalised for GAPDH. (C) Plot showing a significant correlation (Spearman) between the increasing doses of VEGF121 input template and its ratio with GAPDH. (D) Plot showing the mean protein ratios for VEGF/GAPDH. Corresponding Western blots are displayed in E; arrows indicate positive controls.
DISCUSSION
This study shows that VEGF expression, specifically the VEGF121 isoform, is decreased in patients with IDC. The findings were equally apparent in both mild and severe IDC, so this seems an intrinsic quality of IDC. VEGF protein level was also decreased.
Animal studies have supported the possible involvement of VEGF in the pathophysiology and progression of heart failure.6 Additionally, it is well established that in IDC capillary density is decreased, and capillary morphology is altered (luminal swelling, lumen narrowing), and these microvascular abnormalities are thought to play an important role in the perpetuation of heart failure.2 VEGF is a key determinant in capillary growth. Therefore, we propose a concept in which the level of VEGF expression mediates, at least in part, the capillary abnormalities and hence the myocardial contractility in cardiomyopathies, also in the absence of overt coronary artery occlusions. Since we found an equally lowered level of VEGF mRNA expression in mild and severe IDC, the decreased VEGF expression level may represent a mechanism in the progression of IDC.
Thus far, only one report (Abraham and colleagues3) is available on the expression of VEGF in IDC; the present findings are in line with this report with respect to the downregulation of VEGF165 and VEGF189. Abraham and colleagues,3 however, did not investigate the presence of VEGF121, the isoform that we found decreased the most. All three isoforms have angiogenic properties, however the shorter isoforms have been shown to be more potent than VEGF189. The longer VEGF isoforms, especially VEGF189 and to a lesser extent VEGF165, are more tightly bound to the cellular surface and matrix than VEGF121. Given the abundant apposition of matrix-heparin sulfates in IDC, it may be possible that VEGF165 and VEGF189 are accumulated in the matrix, and are less available. VEGF121 that is not bound to the matrix can diffuse more readily in the tissue and may therefore be the most potently mitogenic VEGF isoform in the failing heart.
In conclusion, the results of this study show that the condition of IDC per se leads to decreased expression of VEGF, especially the potent pro-angiogenic isoform VEGF121. VEGF expression is probably not regulated by common cardiac stress pathways, since its decline was not correlated with left ventricular functional parameters. We speculate that interventions that induce angiogenesis, like therapeutic angiogenesis with VEGF121 protein or gene transfer, could be beneficial for patients with IDC.
Acknowledgments
Dr Van Veldhuisen and Dr Tio are supported by the Netherlands Heart Foundation (grants D97-017 and D95-019, respectively).
Abbreviations
IDC, idiopathic dilated cardiomyopathy
GAPDH, glyceraldehyde-3-dehydrogenase
LVEDD, left ventricular end diastolic diameter
LVEF, left ventricular ejection fraction
LVESD, left ventricular end systolic diameter
PCR, polymerase chain reaction
VEGF, vascular endothelial growth factor
REFERENCES
- 1.van den Heuvel AFM, van Veldhuisen DJ, van der Wall EE, et al. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2000;35:19–28. [DOI] [PubMed] [Google Scholar]
- 2.Liu PP, Mak S, Stewart DJ. Potential role of the microvasculature in progression of heart failure. Am J Cardiol 1999;84:23L–26L. [DOI] [PubMed] [Google Scholar]
- 3.Abraham D, Hofbauer R, Schafer R, et al. Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy. Circ Res 2000;87:644–7. [DOI] [PubMed] [Google Scholar]
- 4.Brundel BJ, van Gelder IC, Henning RH, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 2001;103:684–90. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JR, Minton JA, Ho ML, et al. Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin-1beta. J Rheumatol 1997;24:1253–9. [PubMed] [Google Scholar]
- 6.Carmeliet P, Ng YS, Nuyens D, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nature Med 1999;5:495–502. [DOI] [PubMed] [Google Scholar]