Abstract
Objective: To evaluate the effects of the angiotensin converting enzyme inhibitor perindopril on six minute walking distance and quality of life in very old patients with left ventricular systolic dysfunction.
Design: Prospective, double blind placebo controlled trial.
Setting: Medicine for the elderly day hospital.
Patients: 66 patients (average age 81) with left ventricular systolic dysfunction identified by echocardiography.
Interventions: 10 weeks of treatment with titrated doses of perindopril or placebo.
Main outcome measures: Six minute walking distance 10 weeks following treatment, quality of life measurements including the Minnesota living with heart failure questionnaire and the 36 item short form health survey.
Results: In patients with left ventricular systolic dysfunction, six minute walking distance was significantly increased in the treatment group (37.1 m) compared with the placebo group (−0.3 m, p < 0.001). The medication was well tolerated and there were no significant adverse events.
Conclusions: Six minute walking distance is improved considerably by treatment with perindopril in older patients with heart failure caused by left ventricular systolic dysfunction.
Keywords: heart failure, six minute walking distance, elderly, quality of life, perindopril, tolerability
Chronic heart failure is a major health concern and the only major cardiovascular disorder with an increasing incidence and prevalence.1,2 Age itself is a strong predictor for heart failure and most new cases present in elderly patients.3 Heart failure caused by left ventricular systolic dysfunction can be extremely difficult to diagnose in elderly patients because of associated comorbidity.4,5
Clinical trials using angiotensin converting enzyme (ACE) inhibitors have shown clear benefits in the treatment of heart failure caused by left ventricular systolic dysfunction.6,7 Unfortunately, elderly patients have been largely excluded from these studies 8,9 and are underrepresented in the major clinical trials in chronic heart failure.10,11 This has major implications for clinical practice, as the majority of patients seen in day to day clinical practice are elderly and as a group quite different from those patients in the major clinical trials. For the elderly patient, quality of life issues may be as important as any mortality benefit from treatment. The average age of patients recruited to the major trials of chronic heart failure was 61, whereas the median age of patients in Europe with heart failure is 74–76 years.12,13 Elderly patients with heart failure are less likely to be investigated and treated for heart failure.12,14,15 One explanation may be that clinicians are reluctant to extrapolate the findings of the major trials to frail older patients in whom the benefits of treatment are less clear.16 There have been recent calls for further research specifically in elderly patients with heart failure.13,17,18 In response to this call, we have performed a randomised double blind placebo controlled trial to determine the effects of perindopril on exercise capacity, quality of life, and cognitive function in elderly patients with congestive heart failure caused by left ventricular systolic dysfunction.
METHODS
The study was a prospective double blind, randomised, placebo controlled trial. All patients provided written informed consent and the study was approved by the Tayside committee on medical research ethics. Patients were recruited as outpatients by a single centre from four local hospitals, each with day hospital facilities. Patients were eligible for the study if they were aged 70 years or over, they were ACE inhibitor naïve, objective evidence of left ventricular systolic dysfunction on echocardiography was present, and they had a history of symptomatic heart failure. Patients were excluded if they had evidence of significant aortic outflow obstruction, hypotension (systolic blood pressure < 90 mm Hg), serum creatinine concentration > 200 μmol/l, mental score questionnaire results < 6/10, terminal illness, or were wheelchair bound.
Clinical assessment
All patients underwent full clinical assessment and echocardiography. Echocardiographic examination was performed by a single experienced sonographer (SDH) using either a Toshiba Corevision system or Sim 7000 Challenge system. Standard apical and parasternal views were obtained with patients supine in the left lateral position. Left ventricular systolic dysfunction was defined by left ventricular size (> 57 mm), fractional shortening (< 25%), and regional wall motion score (< 1.2). Overall left ventricular function was then qualitatively graded as mild, moderate, or severely impaired. Patients were stabilised on their current cardiovascular treatment and had their fluid balance optimised before randomisation. For each patient, serum biochemistry and full blood count were measured. Six minute walking distance, quality of life, and cognition were assessed using the methods discussed under outcome measures.
Randomisation
Patients were stratified into four convenience sample groups: outpatients in atrial fibrillation (14), outpatients in sinus rhythm (33), day hospital patients in atrial fibrillation (7), and day hospital patients in sinus rhythm (12). Within each stratum, patient packs were randomised to active or placebo by using a restricted randomisation with a block size of four. This was achieved by having the medication supplied prerandomised into blocks of four and sequentially numbered. Separate sets of medication were supplied for each stratum. As patients entered the trial they were allocated medication in sequence in their stratum. Both patients and clinicians were blind, as intervention and placebo packs were identical, medication was in pill form, and treatment and placebo were identical in appearance and taste. Medication was dispensed to patients at study entry and each patient's ability to open the childproof top of the container was assessed. If they had difficulty opening the childproof top they received a standard top.
Test dosing
This was performed in day hospital. After completing baseline outcome measures patients received a test dose, between 10 am and 11 am, of either perindopril 2 mg or placebo and were monitored for the next four hours. Blood pressure (lying and standing) and symptoms were recorded before they took the dose and at 30 minutes, 60 minutes, 120 minutes, 180 minutes, and 240 minutes after.
Follow up
Following randomisation patients were clinically assessed at one week and three weeks and blood pressure, electrolytes, and symptoms were recorded. Patients stable at the three week visit had their dose of medication doubled (to 4 mg perindopril or placebo). At 10 weeks patients returned to day hospital and six minute walking distance, quality of life, and cognitive function were again recorded. The researchers involved in outcome measurements remained blind to treatment allocation throughout the trial. Final blood pressure, electrolytes, and symptoms were recorded. Compliance was assessed by tablet counting. On completion the treatment code was broken by a researcher who was not involved in the day to day conduct of the trial. Each patient's general practitioner was then informed which treatment the patient had received with a note of the patient's progress.
Outcome measures
The primary end point was the change in six minute walking distance over the 10 week treatment period. The six minute walk test has been validated as a safe, reliable, and repeatable measure of functional status and exercise capacity in elderly people.19–23 Tests were conducted using a standardised approach over a 25 m course. Patients used their usual walking aids and received standardised encouragement at regular intervals. The number of stops and total distance walked in six minutes were recorded to the nearest metre. Subgroup analysis was planned to compare the six minute walking distance in day hospital with that of outpatients and to compare patients with mild symptoms (New York Heart Association (NYHA) functional classes I and II) versus those with moderate to severe symptoms (NYHA III and IV). Patients were recruited from all NYHA categories: I-2, II-27, III-35, and IV-2.
Secondary outcome measures were change in quality of life and cognitive function. Quality of life was assessed with the generic 36 item short form (SF-36) health survey and disease specific Minnesota living with heart failure (LIhFE) questionnaire. The SF-36 has been validated as a repeatable measure of health status and is responsive to change in patients with cardiac disorders.24,25 The LIhFE questionnaire has been validated in clinical trials as a measure of change in cardiac status in patients with chronic heart failure.26–28 Both questionnaires were administered by an interviewer (JR) in keeping with previous studies in elderly people.29
Cognitive function was assessed with the Cambridge neuropsychological test automated battery (CANTAB), which uses a computerised touchscreen to assess cognitive function objectively. Tests begin at a simple level and are non-verbal in nature, therefore avoiding problems of language and understanding. CANTAB tests have been established to be sensitive to drug treatments and are repeatable, allowing accurate assessment of cognitive function over a period of time.30,31 Five tests were used to assess cognitive function: motor screening, reaction time, pattern recognition memory, simultaneous and delayed matching to sample, and spatial span.
Statistical analysis
We predicted that a final sample size of 204 recruited over 22 months would have 90% power to detect an increase in six minute walking distance (assuming a standard deviation of 22 m) of 10 m at p < 0.05. A mean distance of 360 m walked in six minutes was anticipated. Sample size estimation allowed for an estimated 20% drop out rate over two months. Data were entered on and analysed using the SPSS-10 statistical package (SPSS, Chicago, Illinois, USA). Change in six minute walking distance and questionnaire scores were normally distributed and analysed using two sample Student's t test. Three of the CANTAB measures had a skewed distribution and were compared using the Mann-Whitney U test (motor screening, reaction time, delayed matching to sample). The other two measures were normally distributed and analysed using two sample Student's t test (pattern spatial recognition memory, spatial span).
RESULTS
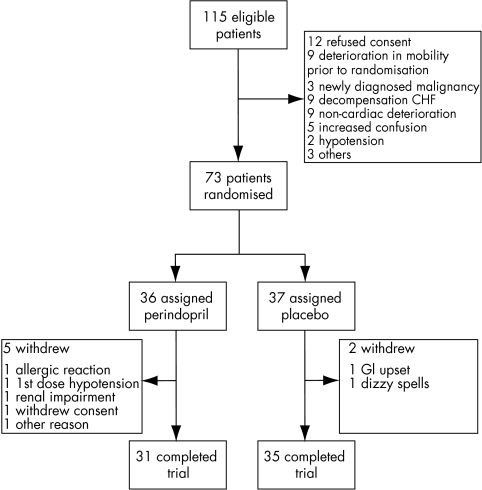
Only 115 potentially eligible outpatients were identified from four hospitals over 22 months from January 1998 to November 2000, less than our originally predicted sample size. Forty two patients were excluded before randomisation for a variety of reasons (fig 1). Seventy three patients were randomly assigned to treatment and 66 patients completed the study. Seven patients withdrew, five on perindopril and two on placebo, but this group did not differ significantly in baseline characteristics from those completing the study. Reasons for withdrawal for the perindopril group were allergic rash (1), test dose hypotension (1), deteriorating renal function (1), gastrointestinal upset (1), and withdrawal of consent (1). In the placebo group they were gastrointestinal upset (1) and a patient unknowingly prescribed ACE inhibitor by a general practitioner before randomisation (1).
Figure 1.
Trial recruitment profile.
Both groups were similar in baseline and demographic variables (table 1). The median age of patients recruited was 81 years (range 68–93 years). Sixty four per cent (42 of 66) of patients were over the age of 80 and 48% (32 of 66) were either in sheltered housing or receiving regular social support. Forty four per cent (29 of 66) required a walking aid, 55% (36 of 66) were prescribed four or more medications, and 15% (10 of 66) could not open the childproof lock and required a normal top. Seventy four per cent (23 of 31) of patients in the treatment group tolerated a dose increase at three weeks compared with 94% (33 of 35) in the placebo group. The reasons for failure to increase dose were hypotension (3), symptoms (4), and deterioration in renal function (1) in the perindopril group, and symptoms (2) in the placebo group. Eighty seven per cent (27 of 31) of patients in the treatment group and 89% (31 of 35) in the placebo group had > 85% compliance with medication assessed by tablet counting.
Table 1.
Demography, patient characteristics, and results summary
| Perindopril (n=31) | Placebo (n=35) | |
| Age ( years) (mean (SD)) | 81.3 (6.2) | 80.7 (5.2) |
| Male:female | 18:13 | 15:20 |
| New York Heart Association functional class | ||
| I/II | 1/15 | 1/12 |
| III/IV | 14/1 | 21/1 |
| Smoking status | ||
| Never | 13 | 18 |
| Former/current | 16/2 | 15/2 |
| Medical history* | ||
| Atrial fibrillation | 12 | 9 |
| IHD | 15 | 21 |
| Hypertension | 8 | 17 |
| Diabetes | 2 | 7 |
| Peripheral vascular disease | 4 | 4 |
| Cerebrovascular accident | 5 | 8 |
| Obstructive airways disease | 8 | 10 |
| Medication | ||
| Prescribed diuretic | 26 | 31 |
| Mean dose furosemide (mg) | 54 | 50 |
| Spironolactone | 2 | 4 |
| Non-steroidal | 1 | 1 |
| Aspirin | 12 | 15 |
| Warfarin | 9 | 6 |
| Use of inhalers | 6 | 11 |
| Digoxin | 13 | 9 |
| β Blocker | 2 | 4 |
| Calcium antagonist | 6 | 9 |
| Nitrate | 1 | 7 |
| Mental score questionnaire (median) | 10 | 9 |
| Left ventricular systolic dysfunction aetiology* | ||
| IHD | 9 | 13 |
| Hypertension | 2 | 5 |
| IHD and hypertension | 6 | 11 |
| Unknown | 14 | 6 |
| Significant valve disease | ||
| Mitral regurgitation | 12 | 15 |
| Tricuspid regurgitation | 6 | 7 |
| Aortic regurgitation | 6 | 9 |
Data are numbers unless otherwise stated.
*Based on patients' history and information from case notes.
IHD, ischaemic heart disease.
Six minute walking distance
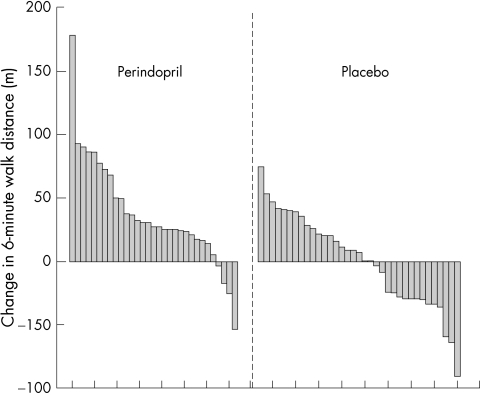
Mean walking distance at baseline in the perindopril and placebo groups was 274 and 277 m, respectively. Patients randomly assigned to perindopril significantly improved their mean walking distance compared with those in the placebo group (p < 0.001) with a mean increase of 37 m in the mean walking distance at 10 weeks. Mean walking distance did not change in the control group (−0.3 m; table 2). Almost all patients in the treatment group improved their walking distance (fig 2).
Table 2.
Outcome measures in the perindopril and placebo groups
| Perindopril (n=31) | Placebo (n=35) | |||||||
| Initial | Final | Change | Initial | Final | Change | 95% CI | p Value | |
| Primary outcome measure | ||||||||
| Mean 6 minute walk distance (m) | 274.8 | 311.9 | 37.1 | 277.3 | 277.0 | −0.3 | 17 to 57 | <0.001 |
| Secondary outcome measures | ||||||||
| Quality of life questionnaires | ||||||||
| Total LIhFE score* | 29.7 | 24.3 | −5.4 | 29.0 | 24.2 | −4.8 | −6.5 to 5.2 | 0.82 |
| Total SF-36 score† | 91.9 | 99.2 | 7.3 | 94.7 | 99.4 | 4.7 | −4.9 to 10.3 | 0.49 |
| CANTAB (cognitive assessment) | ||||||||
| Motor screening time‡ (ms) | 1013 | 947 | −139 | 1031 | 999 | −40 | −178 to 11 | 0.08 |
| Reaction time‡ (ms) | 1013 | 1083 | −38 | 1196 | 1179 | −55 | −73 to 90 | 0.90 |
| Pattern recognition (% correct) | 72 | 75 | 3 | 74 | 72 | −2 | 0 to 11 | 0.05 |
| SDMTS‡ (% correct) | 69 | 72 | 3.4 | 64.5 | 69.5 | 6.7 | −6 to 10 | 0.57 |
| Spatial span (number correct) | 4.2 | 4.6 | 0.4 | 3.8 | 3.8 | 0 | 0 to 0.8 | 0.08 |
Data are mean unless otherwise stated.
* Lower score indicates improvement in symptoms; †higher score indicates improvement in symptoms (no significant difference in any of the eight dimension scores; data not shown); ‡median scores (note that median difference is not the same as the difference in medians).
CANTAB, Cambridge neuropsychological test automated battery; CI, confidence interval; LIhFE, Minnesota living with heart failure; SDMTS, simultaneous and delayed match to sample; SF-36, 36 item short form.
Figure 2.
Change in six minute walking distance for individual patients. Walking distances are in descending order.
Patients who were in NYHA functional class I or II (n = 29) improved significantly with perindopril (36.1 m v 2.0 m, p < 0.05) as did those who were in NYHA III or IV (n = 37) (38.2 m v –1.7 m, p = 0.003). Patients recruited to the study were in all NYHA classes and although there were more patients in NYHA class III in the placebo group, this was not significant in terms of outcome.
Quality of life questionnaires
Both the perindopril and placebo groups' mean SF-36 scores improved slightly after 10 weeks (7.3 and 4.7, respectively). The mean LIhFE scores (5.4 perindopril group, 4.8 placebo group) also improved. However, neither group had any significant change in either the eight dimensions of the SF-36 or the LIhFE total score (table 2).
CANTAB
No significant differences in change in mean scores were detected between the two groups. In three of the five tests—that is, motor screening, pattern recognition, and spatial span tests—the improvement in the perindopril group approached significance (p = 0.08, 0.05, and 0.08, respectively) (table 2).
Blood pressure
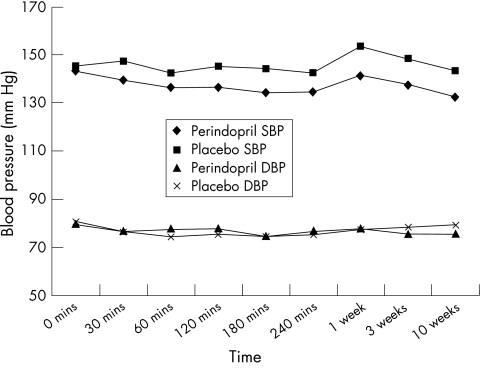
Perindopril was generally well tolerated. Figure 3 summarises the mean blood pressures in each group during the test dose and follow up. Sixteen patients in the perindopril group and 12 in the placebo group had a systolic blood pressure drop of > 20 mm Hg during the four hours following the test dose, all of whom were asymptomatic. Three patients in the treatment group and two in the placebo group had a drop of 40 mm Hg. Only one patient had symptoms with the test dose and had to be withdrawn from the study.
Figure 3.
Mean blood pressure during the test dose and follow up visits.
Renal function
There was no significant change in renal function in either the treatment or placebo groups (table 2). Six patients in each group had a rise in serum creatinine > 25 μmol/l at one point during the study. One patient in the perindopril group had to be withdrawn from the study because of deteriorating renal function (rise in serum creatinine > 40 μmol/l, to 244 μmol/l, with a rise in serum potassium to 5.6 mmol/l). However, this patient had a pre-existing moderate renal impairment (serum creatinine 196 μmol/l) and renal scarring secondary to recurrent urinary tract infections. This person was considered to be at risk of deterioration in renal function before randomisation, and renal function returned promptly to baseline after discontinuing the study medication.
Adverse events
Eight patients in the treatment group and five in the placebo group complained of cough and no patients withdrew form the study because of this. Patients in both groups complained of mild dizziness (four in each group) and mild gastrointestinal upset (two in the treatment group and three in the placebo) but no significant differences were detected between the groups.
DISCUSSION
This is the first randomised placebo controlled trial that assessed the effects of ACE inhibition on six minute walking distance and quality of life in very old patients. Perindopril significantly improved the six minute walking distance compared with placebo but had no effect on quality of life measures. Mean six minute walking distance significantly increased by 37 m (increase of 13.5%) in the treatment group. Perindopril improved the walking distance in both the less symptomatic (NYHA I or II) and the more symptomatic patients (NYHA III or IV). This suggests that elderly patients with mild symptoms may derive just as much improvement in walking distance as the more symptomatic patients.
The quality of life as assessed with the two validated questionnaires did not differ between the two groups. The scores of both groups improved slightly, with the perindopril group showing a greater but non-significant improvement. Although the quality of life questionnaires were the best available they are probably not ideally designed for this cohort of patients. Some of the questions used were not relevant to all older people—for example, questions relating to occupation and sexual activity. The study was powered to detect a change in the primary end point (six minute walking distance) and significantly larger numbers would be required to detect changes in the quality of life measurements.
Cognitive function assessed by CANTAB did show improvements in three tests in the treatment group compared with the placebo group. However, none of these achieved significance. What we can say more securely is that there was no deterioration in cognitive test performance in the treatment group. Cognitive impairment is common in old age and frequently present in elderly patients with cardiac disease.32,33 The finding that ACE inhibitors had no detrimental effects on cognitive function in elderly patients with chronic heart failure is of note. Previous studies in patients with hypertension have suggested that ACE inhibitors may have beneficial effects on cognitive function while other cardiac medications may be detrimental.34–36 Further studies are merited to determine whether treatment with ACE inhibitors results in significant change in cognitive function in older people. The impact of ACE inhibitors on morbidity, including cognitive function, in elderly patients with normal systolic function is being evaluated.37
Treatment was well tolerated with only one patient developing significant hypotension and one other renal impairment. There were no significant symptoms in patients receiving the test dose and no patients had a significant deterioration in renal function. The test dose was given in a day room with the patients sitting in a chair under minimal supervision. The results suggest that perindopril is safe to initiate in the day hospital setting. The lack of problems with this first dose raises the possibility that most such patients may not need any special monitoring, which would facilitate treatment in general practice.
The findings of this study, although novel, should be interpreted cautiously until further data become available. The numbers of patients are relatively small and as such the power of the study is limited. However, the effect on six minute walking distance was highly significant (p < 0.001). Much larger numbers of patients would be required to detect an impact on quality of life and a multicentre study would probably be required.
Before this study there was a remarkable lack of evidence to justify the use of ACE inhibitors in the frail elderly patient with heart failure, despite heart failure being a common, debilitating condition in this group. Reluctance to investigate and use ACE inhibitors in this group of patients may stem from a recognition that very old patients have so many attributes that make them very distinct from patients in the ACE inhibitor megatrials. They have numerous comorbidities and are often already on polypharmacy. Investigation is often minimal because of limited resources in many health care systems. They are thought to suffer more adverse drug effects such as renal dysfunction, dizziness, and hypotension. Finally, improving mortality is not seen as the “prime goal” in treating such patients, which made the ACE inhibitor megatrial findings less relevant to this group. This study firmly dispels such reservations. Despite comorbidity, multiple medications, and extreme age of the patients, perindopril improves the clinically relevant end point of walking distance and does so without producing significant side effects. This study provides a powerful evidence base to justify the widespread use of ACE inhibitors even in very old patients with left ventricular systolic dysfunction.
Acknowledgments
This project was funded by an education grant from Servier Laboratories Ltd. We thank Mrs Jess Robson (research sister) for patient assessment and data collection. The assistance of the staff at Dundee, Monifieth, Perth, and Stracathro Day Hospitals is gratefully acknowledged. We also thank the Dundee Levi-Strauss Community Action Group for providing the funds to purchase the portable echocardiography machine. Finally we thank the patients who participated in the study for their time and assistance with the study.
Abbreviations
CANTAB, Cambridge neuropsychological test automated battery
LIhFE, Minnesota living with heart failure
NYHA, New York Heart Association
SF-36, 36 item short form
REFERENCES
- 1.Anon. Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure. Am J Cardiol 1999;83:1A–38A. [PubMed] [Google Scholar]
- 2.Hoes AW, Mosterd A, Grobbee DE. An epidemic of heart failure? Recent evidence from Europe. EurHeart J 1998;19(suppl L):L2–9. [PubMed] [Google Scholar]
- 3.Cowie MR, Wood DA, Coats AJ, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421–8. [DOI] [PubMed] [Google Scholar]
- 4.Wheeldon NM, MacDonald TM, Flucker CJ, et al. Echocardiography in chronic heart failure in the community. Q J Med 1993;86:17–23. [PubMed] [Google Scholar]
- 5.Remes J, Miettinen H, Reunanen A, et al. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315–21. [DOI] [PubMed] [Google Scholar]
- 6.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative group on ACE inhibitor trials [published erratum appears in JAMA 1995 Aug 9;274:462]. JAMA 1995;273:1450–6. [PubMed] [Google Scholar]
- 7.Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-inhibitor myocardial infarction collaborative group. Lancet 2000;355:1575–81. [DOI] [PubMed] [Google Scholar]
- 8.Willenheimer R, Dahlof B, Gordon A. Clinical trials in cardiovascular medicine: are we looking for statistical significance or clinical relevance? Heart 2000;84:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lye M. Access to advances in cardiology. Lancet 1997;350:1162–3. [DOI] [PubMed] [Google Scholar]
- 10.Bugeja G, Kumar A, Banerjee AK. Exclusion of elderly people from clinical research: a descriptive study of published reports. BMJ 1997;315:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avorn J. Including elderly people in clinical trials. BMJ 1997;315:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Solal A, Desnos M, Delahaye F, et al. A national survey of heart failure in French hospitals. The myocardiopathy and heart failure working group of the French Society of Cardiology, the National College of General Hospital Cardiologists and the French Geriatrics Society. Eur Heart J 2000;21:763–9. [DOI] [PubMed] [Google Scholar]
- 13.Cleland JG, Swedberg K, Poole-Wilson PA. Successes and failures of current treatment of heart failure. Lancet 1998;352(suppl I):SI19–28. [DOI] [PubMed] [Google Scholar]
- 14.Gambassi G, Forman DE, Lapane KL, et al. Management of heart failure among very old persons living in long-term care: has the voice of trials spread? The SAGE study group. Am Heart J 2000;139:85–93. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs FD. Primary care physicians: champions of or an impediment to optimal care of the patient with heart failure? Eur J Heart Fail 1999;1:11–5. [DOI] [PubMed] [Google Scholar]
- 16.Remme WJ. Heart failure management: why evidence does not influence clinical practice. Eur Heart J 2000;2(suppl I):115–21. [Google Scholar]
- 17.McMurray JJ. Failure to practice evidence-based medicine: why do physicians not treat patients with heart failure with angiotensin-converting enzyme inhibitors? Eur Heart J 1998;19(suppl L):L15–21. [PubMed] [Google Scholar]
- 18.McMurray J. Heart failure: we need more trials in typical patients. Eur Heart J 2000;21:699–700. [DOI] [PubMed] [Google Scholar]
- 19.Willenheimer R, Erhardt LR. Value of 6-min-walk test for assessment of severity and prognosis of heart failure. Lancet 2000;355:515–6. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 21.Poole-Wilson PA. The 6-minute walk. A simple test with clinical application. Eur Heart J 2000;21:507–8. [DOI] [PubMed] [Google Scholar]
- 22.O'Keeffe ST, Lye M, Donnellan C, et al. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart 1998;80:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters P, Mets T. The 6-minute walk as an appropriate exercise test in elderly patients with chronic heart failure. J Gerontol A Biol Sci Med Sci 1996;51:M147–51. [DOI] [PubMed] [Google Scholar]
- 24.Garratt AM, Ruta DA, Abdalla MI, et al. SF 36 health survey questionnaire. II. Responsiveness to changes in health status in four common clinical conditions. Qual Health Care 1994;3:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry C, McMurray J. A review of quality-of-life evaluations in patients with congestive heart failure. Pharmacoeconomics 1999;16:247–71. [DOI] [PubMed] [Google Scholar]
- 26.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993;71:1106–7. [DOI] [PubMed] [Google Scholar]
- 27.Rogers WJ, Johnstone DE, Yusuf S, et al. Quality of life among 5,025 patients with left ventricular dysfunction randomized between placebo and enalapril: the studies of left ventricular dysfunction. The SOLVD Investigators. J Am Coll Cardiol 1994;23:393–400. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH. Measurement of health-related quality of life in heart failure. J Am Coll Cardiol 1993;22(4 suppl A):185A–91A. [DOI] [PubMed] [Google Scholar]
- 29.Hayes V, Morris J, Wolfe C, et al. The SF-36 health survey questionnaire: is it suitable for use with older adults? Age Ageing 1995;24:120–5. [DOI] [PubMed] [Google Scholar]
- 30.Robbins TW, James M, Owen AM, et al. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–81. [DOI] [PubMed] [Google Scholar]
- 31.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein RA, Starling RC, Myerowitz PD, et al. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand 1995;91:260–5. [DOI] [PubMed] [Google Scholar]
- 33.Pathy SJ. Hypertension and associated diseases in elderly patients. J Hypertens Suppl 1988;6:S37–40. [PubMed] [Google Scholar]
- 34.McDevitt DG, Currie D, Nicholson AN, et al. Central effects of repeated administration of atenolol and captopril in healthy volunteers. Eur J Clin Pharmacol 1994;46:23–8. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson AN, Wright NA, Zetlein MB, et al. Central effects of the angiotensin-converting enzyme inhibitor, captopril. II. Electroencephalogram and body sway. Br J Clin Pharmacol 1990;30:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olajide D, Lader M. Psychotropic effects of enalapril maleate in normal volunteers. Psychopharmacology (Berl) 1985;86:374–6. [DOI] [PubMed] [Google Scholar]
- 37.Cleland JG, Tendera M, Adamus J, et al. Perindopril for elderly people with chronic heart failure: the PEP-CHF study. The PEP investigators. Eur J Heart Fail 1999;1:211–7. [DOI] [PubMed] [Google Scholar]