Abstract
Objective: To investigate the relative importance of stent induced arterial stretch and deep injury to the development of in-stent neointima.
Setting: Normal porcine coronary arteries
Methods: 30 BiodivYsio stents (Biocompatibles) were deployed at a stent to artery ratio of 1.25:1 (a moderate injury) and harvested at 28 days. Multiple serial cross sections were analysed morphometrically and the neointimal areas were correlated with the type and degree of injury.
Results: Arterial stretch occurred in 78% of struts (77% of sections) and produced moderate neointimal growth (neointimal area 1.93 (0.13) mm2). Deep injury (rupture of the internal elastic lamina) occurred in 20% of struts (23% of sections) and produced a 1.7-fold increase in neointimal area (3.33 (0.41) mm2) compared with stretch only (p = 0.0002). With even deeper injury (rupture of the external elastic lamina), there was a 2.6-fold increase in neointimal area (5.01 (0.48) mm2) compared with stretch only (p = 0.02). A new injury score, incorporating both stretch and deep injury, correlated with neointimal area (r = 0.60, p < 0.001).
Conclusions: Stretch of the coronary artery in a stent is common, and a major contributor to neointima formation, even in the absence of deep injury. Deep injury is, however, a more potent stimulus to neointima formation than stretch. Greater degrees of stretch are associated with thicker neointima. Where neither deep injury nor stretch are seen, the stent has no effect upon the development of neointima.
Keywords: stent injury, arterial stretch, neointima, restenosis
Stents are now used in more than 70% of percutaneous coronary interventions. In-stent restenosis is important because it is common and difficult to treat. It is known from intravascular ultrasound (IVUS) studies that in-stent dimensions and stent length are the main predictors of in-stent restenosis.1 Immediate contributors to in-stent restenosis include recoil (particularly in coil stents) and underexpansion.2,3 Multicellular designs appear to produce less vascular injury and neointima than slotted tubes.4 High pressure deployment, wide strut openings, asymmetrical deployment, and increased balloon compliance may also contribute to in-stent restenosis or experimental neointima formation.5–8 The balance to be achieved is, therefore, in attaining adequate final stent dimensions without an excess of vascular injury, because vascular injury is intimately linked to in-stent neointima formation.9 Stent imposed injury (unlike balloon injury) is persistent, and it may worsen with time.10 Vascular injury may be seen as deep penetration of the arterial wall leading to medial rupture.9 Deep injury is likely to be important if stent implantation is aggressive; but with more moderate implantation, the contribution of stretch to neointima formation may be underestimated.
Our aim in this study was to investigate the relative importance of arterial stretch and deep injury to the late arterial response after stent deployment, using moderate implantation conditions.
METHODS
Stents
The BiodivYsio stent used in this study is 15 mm long and laser cut from a tube of 316L electropolished stainless steel with an “interlocking arrowhead” design incorporating S shapes for flexibility. It is coated with a phosphorylcholine containing polymer and is premounted on a 3.5 mm balloon. The coating has been shown by us to have no effect on neointima formation in this model.11
Stent implantation
The experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986. Yorkshire White pigs (15–20 kg) were used. General anaesthesia was induced with azaperone 12 mg/kg and maintained with inhaled enflurane 5% in oxygen using an endotracheal tube. Vascular access was gained through the carotid artery. Under fluoroscopic guidance, with ECG monitoring, a 6 French Amplatz right coronary guide catheter was used to intubate both coronary arteries. Quantitative angiography was performed in each of the right and left coronary arteries. Stent implantation sites were chosen so that the balloon to artery ratio was 1.25:1 (a stent mounted on a 3.5 mm balloon placed in a 2.8 mm segment of artery). Balloon inflation was at 8 atm for 30 seconds (to produce a moderate injury). Heparin (2500 U) was given intravenously before implantation, and oral aspirin 150 mg orally for five days, starting on the day of implantation. An angiogram was obtained at the end of the procedure to document arterial patency.
Processing of stented arteries
The animals were killed by an intravenous injection of sodium pentobarbitone (pentobarbital) at 28 days. The hearts were explanted and the stented segments of artery flushed with saline, removed, and immersion fixed in 4% buffered formalin for 24 hours. The stented arteries were cut, ground, and polished using methods published by our group, in which the metal remains in situ and the tissue is undamaged, enabling accurate assessment of tissue morphometry without tissue damage.12 Multiple sections were cut from each stent. Up to three sections were selected from each stent—from the proximal, middle, and distal thirds. Other sections were rejected on the grounds of an incomplete ring of struts, a large branch, distortion, tissue loss, or proximity to the end of the stent. Two or three good quality representative sections were thereby obtained from each stent.
Measurement of deep injury
Two measures of deep injury were made: rupture of the internal elastic lamina, and rupture of the external elastic lamina (the latter implying rupture of the media).
Injury scores
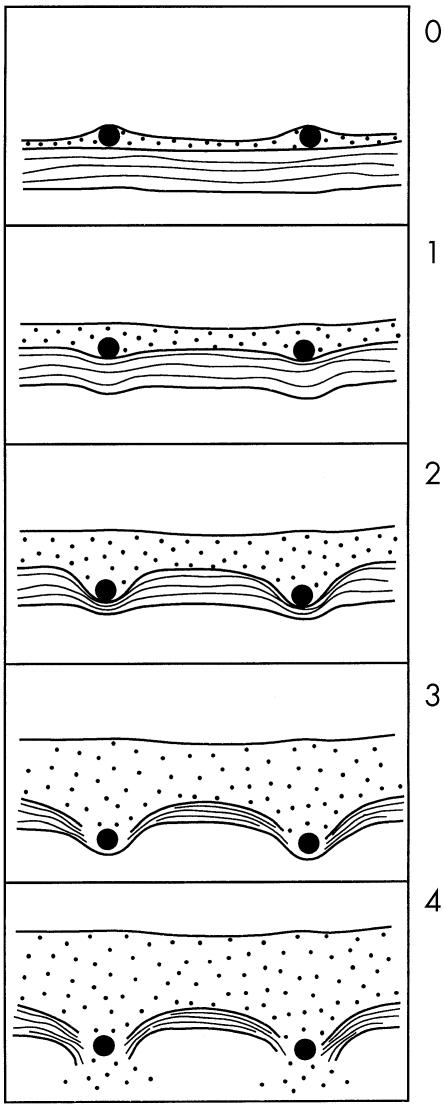
Two previously described injury scores were measured: the “deep injury” Schwartz score,13 and the “media compression” score of Waale.14 Briefly, the Schwartz score assesses the presence or absence of rupture of the internal elastic lamina, the media, and the external elastic lamina, and the Waale score assesses the degree of angular deformation of the media by the struts. In addition, to include both deep injury and stretch, a novel score was constructed as follows: 0, no impression of metal upon media; 1, deformation of the internal elastic lamina by < 45°; 2, deformation of the internal elastic lamina by > 45°; 3, rupture of the internal elastic lamina; 4, rupture of the external elastic lamina (that is, complete medial rupture). The score for each section was the mean of the individual strut scores (fig 1).
Figure 1.
New injury score, embracing all grades of stent induced injury, from none (0), through stretch (1 and 2), to deep injury (3 and 4). 0, no deformation; 1, IEL deformed < 45°; 2, IEL deformed > 45°; 3, ruptured IEL; 4, ruptured EEL. EEL, external elastic lamina; IEL, internal elastic lamina.
Arterial morphometry
Sections were analysed by computed morphometry. The cross sectional areas of the lumen, neointima, media, adventitia, stent, and whole vessel were recorded. Neointimal thicknesses (defined as the minimum distance between the strut and the lumen) were also measured.
Statistical analysis
Descriptive statistics (vessel cross sectional areas and thicknesses) are expressed as mean (SEM). Comparisons between groups were made with unpaired t tests. The relation between each injury score and the measurements of the lumen, neointima, and arterial wall layers were investigated with product–moment correlation coefficients. A probability value of p < 0.05 was regarded as significant.
RESULTS
Stent implantation and processing
Thirty BiodivYsio stents were deployed in 15 pigs, 15 in the left anterior descending coronary artery and 15 in the right coronary artery. All stents were angiographically patent after implantation. The achieved balloon to artery ratio, determined by quantitative coronary angiography, was 1.23 (0.02) to 1. From the 30 stents harvested at 28 days, we obtained 77 sections (a mean of 2.6 per stent) which fulfilled the criteria described above.
Stent related injury
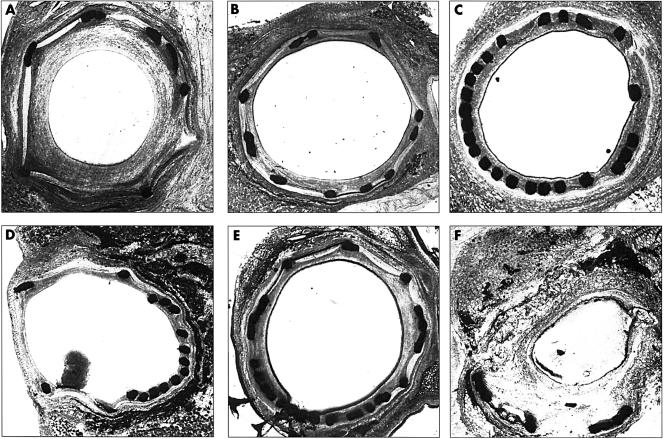
Sections displaying different types of stent related injury are shown in fig 2. Four examples of stretch and two of deep injury are shown. Sections displaying varying degrees of stent related injury are shown in fig 3.
Figure 2.
Types of stent induced injury. (A) Circumferential oversize plus large interstrut distances between 3 and 9 o'clock have led to stretch of the arterial wall and moderate circumferential neointima formation. There is one deep injury, where the strut at 4 o'clock has penetrated both the IEL and the EEL. Neointima formation is not confined to the area immediately around each strut. (B) A typical section with mild oversize, stretch, and neointima, despite similar interstrut distances to (A). (C) Mild oversize with more struts than (B), but similar neointima; the stent is therefore not a direct stimulus to neointima formation. (D) Almost no stretch or neointima, despite gross strut protrusion between 7 and 12 o'clock. Geometrical variations in the stent thus only lead to neointimal growth in the presence of stretch. (E) Despite a strut distribution similar to (D), the addition of mild stretch drives mild neointima formation. (F) Oversize (distal end of a stent) with destruction of both the internal and external elastic laminae; obvious deep injury rather than stretch. Neointimal growth is concentric and considerable. EEL, external elastic lamina; IEL, internal elastic lamina.
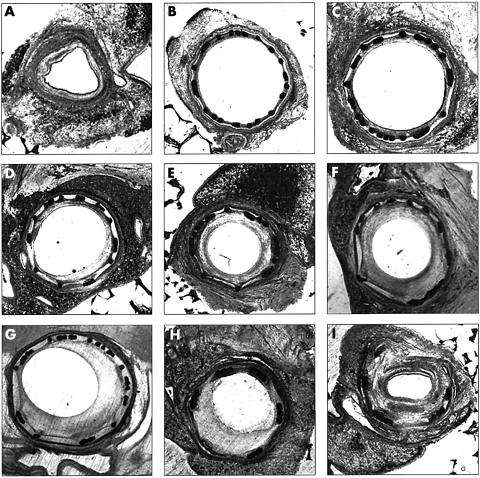
Figure 3.
Grades of injury, from mild stretch to deep injury. (A) A control (unstented) artery. (B, C) Almost 1:1 sizing (proximal end of stent) and even strut deployment lead to mild stretch and minimal neointimal growth. (D–G) Progressively more stretch is associated with a proportionately larger neointima. (H, I) Ruptured IEL affecting half (H) and the whole (I) vessel leads to pronounced neointimal growth. IEL, internal elastic lamina.
Measures of stretch and deep injury
Twenty per cent of struts (23% of sections) displayed deep arterial injury. Rupture of the internal elastic lamina was present in 14% of sections, and of the external elastic lamina in 9%. Seventy eight per cent of struts (77% of sections) showed evidence of arterial stretch without deep injury; 2% of struts (0% of sections) showed no stretch.
Injury scores
The Schwartz score was 0.23 (0.06). The Waale score was 0.42 (0.02). The new score was 1.74 (0.09).
Arterial morphometry
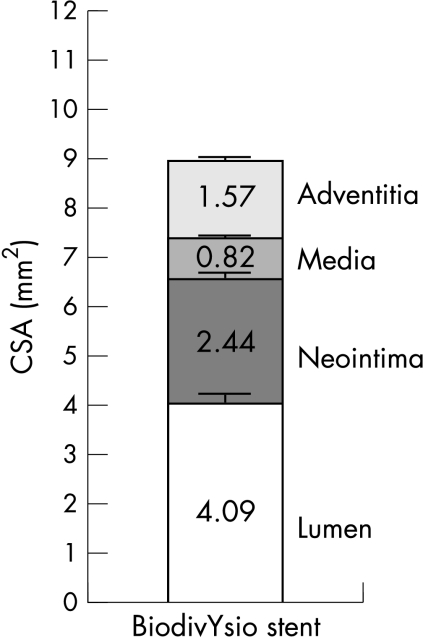
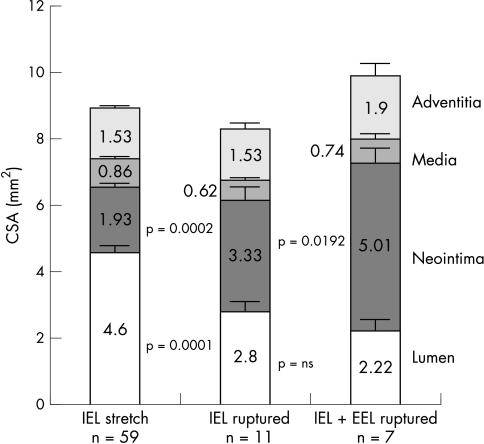
The quantitative morphometry of the wall layers of all 77 sections is shown in fig 4.
Figure 4.
Quantitative morphometry of the BiodivYsio stent 28 days after implantation at a balloon/artery ratio of 1.25:1. Data derived from 77 stent sections evenly spaced through 30 stents. CSA, cross sectional area.
Deep injury and neointimal area
In sections containing a rupture of the internal elastic lamina, neointimal cross sectional area was 1.7-fold greater than in those in which there was no deep injury (p = 0.0002). In sections containing a rupture of the external elastic lamina, neointimal cross sectional area was 1.5-fold greater than in those with internal elastic lamina rupture only (p = 0.02) (fig 5).
Figure 5.
Effect of deep injury, as assessed by rupture of the internal elastic lamina (IEL) and (in the more extreme case) of the external elastic lamina (EEL) upon neointima formation.
Deep injury and remodelling
The total vessel cross sectional area was 8.91 (0.19) mm2 in arteries with stretch only, whereas arteries containing an internal elastic lamina rupture had a 7% smaller total cross sectional area (downsize remodelling, p = 0.19) and those with an external elastic lamina rupture an 11% larger total cross sectional area (upsize remodelling, p = 0.08) (fig 5).
Injury score and neointimal area
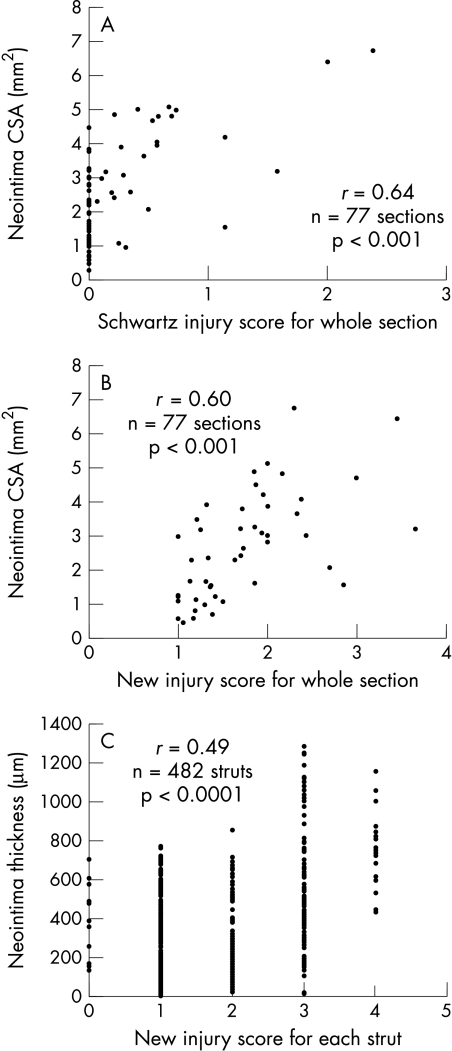
The correlation between the three injury scores and neointimal cross sectional areas were: Schwartz, r = 0.64 (p < 0.0001); Waale, r = 0.43 (p = 0.001); and new score, r = 0.60 (p < 0.001) (fig 6).
Figure 6.
Correlations between injury scores and neointimal growth at 28 days. (A) and (B) show aggregate scores for the whole section versus neointima area: (A) Schwartz score; (B) a new score. Note the clustering around zero with the Schwartz score. There is better spread with the new score, which adequately represents stretch. (C) Individual neointimal thickness at each strut plotted against new scores for each strut. There is a positive correlation but it is no stronger than that between the injury score for the whole section and total neointimal area.
Injury score and neointimal thickness
The correlation between the new injury score (for each strut) and neointimal thickness (for each strut) was r = 0.49 (p < 0.001) (fig 6).
DISCUSSION
In this study we showed that stretch of the media leads to neointima formation even in the absence of deep injury. With moderate stent oversize, stretch is more common than deep injury and is associated with neointimal growth. The greater the stretch, the larger the neointima. Deep injury, where it occurs, is a more powerful stimulus to neointima formation and is associated with a larger neointima than stretch alone. Rupture of the external elastic lamina (a deeper structure than the internal elastic lamina) is a yet more potent stimulus than rupture of the internal elastic lamina alone. A new injury score, devised to include all grades of stretch and deep injury, correlates well with neointima formation. Because of its universality, stretch might be considered to be the most important overall stimulus to in-stent restenosis where vascular injury is moderate.
We divided deep injury into rupture of the internal elastic lamina and rupture of both internal elastic lamina and external elastic lamina. This distinction appeared to be of biological importance (fig 5), rupture of the deep layer providing a further boost to neointima formation. Occasionally, deep injury could be associated with a single strut protruding from its neighbours (fig 2A), but it also occurred where there was overall oversizing (fig 2F). Stretch injury was synonymous with deformation of the circular artery by the polygonal stent. It occurred in almost every section examined, even in those with some localised deep injury.
Where there was no tension exerted upon the arterial wall, there was no neointimal growth (fig 3B). This is an important point. It implies (1) that a phosphorylcholine coated stent per se is completely biologically neutral and elicits no reaction from the artery; and (2) that stent geometry is only important in the presence of stretch.
In our model, with scant deep injury, the Schwartz score was low and did not differentiate between sections with mild and extreme strut impaction. There was thus a pronounced clustering of neointimal responses around a zero Schwartz score (fig 6A), although enough data points were left to provide a significant positive relation between deep injury and neointimal response. The Waale score provided a better data spread but showed a less intimate relation between injury—as assessed by media compression—and neointima formation than the Schwartz score. The new injury score included all grades of injury, and showed both good data spread and a close relation between injury and neointimal cross sectional areas (fig 6B). This score might therefore be more appropriate for the analysis of stented segments exposed to moderate injury. It would also allow correction for the degree of injury in the experimental setting.
The relation between the new injury score for the whole section and the neointimal cross sectional area was closer than the relation between the new injury score for each strut and the neointimal thickness at each strut (fig 6Bv 6C). This suggests that local stretch has effects not confined to the region immediately around the offending strut, but transmitted to the whole artery. This is an argument in favour of measuring arterial cross sectional area in stent studies rather than the maximum or average neointimal thickness. More importantly, cross sectional area reflects clinical restenosis better than a point measurement of intimal thickness.
There are two possible mechanisms whereby stretch might lead to neointima formation independent of deep injury. First, impaction of the stent causes compression of the vascular smooth muscle cells (VSMC) in the media beneath each strut. With major strut penetration, even with intact elastic laminae, this may cause atrophy of the media, although where cells abut the strut it is difficult to identify the presence or absence of nuclei. Cell death (necrosis or apoptosis) might then start a biological process leading to neointima formation. Second, stretch may transduce biological events directly. Stretch is transmitted cell to cell by adhesion molecules, and transduced through the cell by intracytoplasmic integrins, which may activate kinase signalling pathways leading to cell proliferation15 or matrix metalloproteinases leading to connective tissue production.16 The suggestion that neointima might occur in response to stent geometry, independent of injury,17 might now be explicable by the presence of stretch deformation.
There was a trend towards downsize remodelling of the whole vessel where there was a ruptured internal elastic lamina, whereas there was a trend towards upsize remodelling in the presence of a ruptured external elastic lamina. This may be explained by the constraining effect of an intact external elastic lamina allowing only inward expansion of neointima and loss of this constraint allowing inward and outward expansion of tissue. Stents have always been thought to eliminate remodelling, but our data suggest that, while this may be the case for deep injury where tissue growth can be outwards, it may not be where the injury is mild.
Limitations
There were some limitations of this study. First, the arteries were undiseased. On the other hand, undiseased arteries reveal the effect of subtle variations in stent geometry, independent of the wide variations inherent in disease models. Second, the vessels were porcine, not human. The pig is, however, the closest practical model with anatomical similarity to man. Third, arterial morphometry may change after 28 days. This time point was chosen as a practical compromise between completeness of neointima formation and the costs of animal husbandry.
Conclusions
Arterial stretch commonly follows from stent implantation, and is enough to stimulate neointima formation, even in the absence of deep injury, although deep injury is a more potent stimulus. A new injury score, including all injuries from mild stretch to deep penetration, works in this model. The effects of stretch are manifested more in the total (circumferential) amount of neointima than in its thickness at any one point. Without stretch, there is no neointima; thus stent geometry is only important in the presence of stretch. These findings may contribute to developing improved stent designs and stent related drug delivery systems.
Acknowledgments
We are grateful to the Special Trustees of the Former United Sheffield Hospitals' Charitable Funds and to Biocompatibles Ltd, Farnham, UK for their support of this study.
Conflict of interest: Dr Gunn performs consultancy work for Biocompatibles
REFERENCES
- 1.de Feyter PJ, Kay P, Disco C, et al. Reference chart derived from post-stent-implantation intravascular ultrasound predictors of 6-month expected restenosis on quantitative angiography. Circulation 1999;100:1777–83. [DOI] [PubMed] [Google Scholar]
- 2.Carter AJ, Scott D, Rahdert D, et al. Stent design favourably influences the vascular response in normal porcine coronary arteries. J Invas Cardiol 1999;11:127–34. [PubMed] [Google Scholar]
- 3.Carozza JP, Hosley SE, Cohen DJ, et al In vivo assessment of stent expansion and recoil in normal porcine coronary arteries. Differential outcome by stent design. Circulation 1999,100:756–60. [DOI] [PubMed] [Google Scholar]
- 4.Schulz C, Herrmann RA, Rybnikar A, et al. Experimental results with a new gold-coated multicellular stent design: comparison with a conventional slotted tube stent in the coronary overstretch model of the pig. J Intervent Cardiol 1999;12:191–8. [Google Scholar]
- 5.Rogers C, Tseng DY, Squire JC, et al. Balloon–artery interactions during stent placement – a finite element analysis approach to pressure, compliance and stent design as contributors to vascular injury. Circ Res 1999;84:378–83. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SL, DiMario C, Hall P, et al. Comparison of aggressive vs nonaggressive balloon dilatation for stent deployment on late loss and restenosis in native coronary arteries. Am J Cardiol 1998,81:708–12. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann R, Mintz GS, Mehran R, et al. Tissue proliferation within and surrounding Palmaz-Schatz stents is dependent on the aggressiveness of stent implantation technique. Am J Cardiol 1999,83:1170–4. [DOI] [PubMed] [Google Scholar]
- 8.Schulz C, Herrmann RA, Beilharz C, et al. Coronary stent asymmetry and vascular injury determine experimental restenosis. Heart 2000,83:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–74. [DOI] [PubMed] [Google Scholar]
- 10.Hofma SH, Whelan DMC, van Beusekom HMM, et al. Increasing arterial wall injury after long-term implantation of two types of stent in a porcine coronary model. Eur Heart J 1998;19:601–9. [DOI] [PubMed] [Google Scholar]
- 11.Malik N, Gunn J, Holt C, et al. Phosphorylcholine-coated stents in porcine coronary arteries: biocompatible and safe. J Invas Cardiol 2001;13:193–201. [PubMed] [Google Scholar]
- 12.Malik N, Gunn J, Holt C, et al. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry and transmission electron microscopy. Heart 1998;80:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz RS, Holmes DR. Pigs, dogs, baboons and man: lessons for stenting from animal studies. J Intervent Cardiol 1994;7:355–68. [Google Scholar]
- 14.Waale WHE, van der Veen FH, van Leeuwen C, et al. Modulation of healthy pig coronary arteries by self-expanding stents. J Intervent Cardiol 1996;9:45–52. [Google Scholar]
- 15.Zou Y, Hu Y, Metzler B, et al. Signal transduction in arteriosclerosis: mechanical stress-activated MAP kinases in vascular smooth muscle cells. Int J Mol Med 1998;1:827–34. [DOI] [PubMed] [Google Scholar]
- 16.Yang JH, Briggs WH, Libby P, et al. Small mechanical strains selectively suppress matrix metalloproteinase-1 expression by human vascular smooth muscle cells. J Biol Chem 1998;273:6550–5. [DOI] [PubMed] [Google Scholar]
- 17.Garasic JM, Edelman ER, Squire JC, et al. Stent and artery geometry determines intimal thickening independent of arterial injury. Circulation 2000;101:812–18. [DOI] [PubMed] [Google Scholar]