Abstract
Background: Carotid artery stenting is now used as an alternative to surgical endarterectomy. The availability of cerebral protection systems has expanded the area of application of this procedure.
Objective: To assess the feasibility, safety, and immediate and late clinical outcome in patients undergoing percutaneous carotid interventions.
Methods: Between January 1999 and December 2000, 100 consecutive patients with 102 carotid artery stenoses were treated (71 men, 29 women, mean (SD) age 67 (8) years): 49 had coronary artery disease, 28 had previous stroke or transient ischaemic attack (TIA). On the basis of the Mayo Clinic carotid endarterectomy risk scale, 73 patients were grade III–IV and 13 grade VI.
Results: Baseline diameter stenosis was 78.8 (10)%, with a mean lesion length of 12.6 (5.8) mm. Angiographic success was obtained in 99 lesions (97.0%) with a final diameter stenosis of 2.4 (3.5)%. Procedural success was obtained in 96 patients (96%). Selective cannulation of three carotid arteries was impossible owing to severe vessel tortuosity. Carotid stenting was performed in 97 of the treated lesions, and protection devices were used in 67 lesions. In-hospital complications occurred in seven patients (six TIA, one (category 1) minor stroke). No major stroke or death occurred. All patients were discharged from the hospital after an average of 2.5 days. At 12 (6.2) months of follow up restenosis occurred in three patients (3.4%) (one patient with carotid occlusion had TIA). Six patients had died: two from cerebrovascular events (5 and 11 months after the procedure) and four from cardiovascular causes.
Conclusions: Carotid stenting appears feasible and safe, with few major complications. Long term follow up is affected by a high incidence of cardiovascular mortality.
Keywords: carotid artery disease, carotid stenting
Carotid endarterectomy has been shown to be superior to medical treatment in reducing the overall risk of stroke in symptomatic or asymptomatic patients with significant carotid artery stenosis.1,2
Although endarterectomy is considered to be the gold standard treatment for carotid artery stenosis, the approach is not free of complications. In the NASCET (North American symptomatic carotid endarterectomy trial) study population, 5.8% of patients suffered from perioperative stroke and death, and it was also reported that subgroups of patients at high risk had mortality and morbidity rates of up to 18%.1,3–5 Since the first percutaneous transluminal carotid angioplasty (PTA) was performed by Kerber in 1980,6 rapid improvements in interventional technology and materials have contributed to the increasing popularity of this technique.
PTA with carotid stenting (PTACS) has led to the achievement of optimal immediate angiographic results and has become a standard percutaneous approach to carotid artery stenosis. Acceptable rates of immediate complications (particularly in patients at high surgical risk) have been reported in several studies, with good long term results after carotid stenting.7–13 In addition, the recent introduction of distal protection devices has lowered the rate of periprocedural acute cerebral ischaemic complications, thus enhancing the safety of the percutaneous approach, which can thus be performed with good results even in high risk patients.12
In this paper we present our experience of the feasibility, safety, and immediate and late clinical outcome in the first 100 consecutive patients who underwent percutaneous carotid intervention in our institutions.
METHODS
Patient population
Between January 1999 and December 2000, 100 consecutive patients (71 male, 29 female) with 102 lesions underwent carotid angioplasty at the Division of Interventional Cardiology, IRCCS, San Raffaele and the EMO Centro Cuore, Columbus Hospital, Milan, Italy.
Patients with angiographic evidence of ≥ 60% diameter stenosis were selected for treatment.1,2,7 The decision to treat these patients by the percutaneous approach was taken on the basis of whether or not there were contraindications to surgical treatment and according to the patient's or referring physician's preference.
A neurologist evaluated the neurological status of all the patients before and after the procedure. Only those patients with previous ischaemic cerebral events (stroke/transient ischaemic attack) were considered symptomatic. Cerebral computed tomography was undertaken only in patients with previous neurological events. All patients were on combined antiplatelet treatment with aspirin (mean dose 100 mg/day) and either ticlopidine (250 mg twice a day) or clopidogrel (75 mg once a day), starting at least 48 hours before the procedure. Ticlopidine or clopidogrel were continued for at least one month after the procedure if a stent was implanted.
Definitions
Transient ischaemic attack (TIA)
—temporary focal cerebral or retinal deficits that resolved within 24 hours.
Minor stroke category 1
—a new neurological deficit that persisted for a period of more than 24 hours but resolved completely or returned to baseline within seven days, and changed the National Institutes of Health stroke scale (NIHSS)14,15 by 1 point; non-disabling event, Rankin index < 3.
Minor stroke category 2
—a new neurological deficit that either resolved completely or returned to baseline within 30 days, or that changed the NIHSS by 2 or 3 points; non-disabling event, Rankin index < 3.
Major stroke
—a new neurological deficit that persisted after 30 days and that changed the NIHSS by ≥ 4 points; disabling, Rankin index > 3.
Myocardial infarction
—development of a new Q wave on the ECG according to the Minnesota code, and/or an increase in creatine kinase (CK) to at least twice the upper limit of normal, associated with above normal elevation of CK-MB isoenzymes.
Angiographic success
—less than 30% residual diameter stenosis by quantitative coronary angiographic analysis; procedural success was defined as angiographic success and freedom from occurrence of death, new neurological events lasting more than 24 hours (any neurological event except for TIA), myocardial infarction, or major bleeding up to the time of discharge from hospital.
Restenosis severity
—assessed by ultrasound evaluation according to the conventional Doppler criteria: maximum velocity (Vmax) < 80 cm/s detects a < 40% stenosis; Vmax from 80–175 cm/s detects a 40–80% stenosis; Vmax > 175 cm/s detects a 80–99% stenosis.16
All patients received detailed information about potential risks and benefit of the procedure and signed an informed consent form which had previously been approved by the ethics committee at our institutions
Procedure
Under local anaesthesia, percutaneous access was gained with the Seldinger technique through the right femoral artery or, rarely, through the left femoral artery. A bolus of unfractionated heparin (70 IU/kg) was given intravenously; further boluses were given as needed to maintain the activated clotting time between 200–250 seconds. Blood pressure and ECG were monitored continuously. Glyceryl trinitrate, atropine, and positive inotropic drugs were also readily available. Severe bradycardia and hypotension were defined as a heart rate of ≤ 40 beats/min and a systolic blood pressure of ≤ 90 mm Hg, respectively.
Angiography of the extracranial carotid systems, as well as of the intracranial circulation, was done routinely in standard projections. The patient's neurological status was monitored constantly throughout the procedure.17
Where a distal protection device was used, predilatation with a 2.5 mm coronary balloon was undertaken only when the device could not be advanced through the lesion as a primary manoeuvre.
Self expandable stents were deployed and then postdilated with a balloon according to the size of the internal carotid artery.
We routinely gave 1 mg of atropine intravenously before balloon dilatation to prevent pronounced sinus bradycardia.
Angiographic analysis
Angiograms were analysed by a semiautomated edge contour detection computer analysis system (MEDIS, QCA-SMS version 4). Reference diameter, minimum lumen diameter, and per cent diameter stenosis were measured before and at the end of the procedure, using the NASCET angiographic measurement criteria.18
Statistical analysis
We used the StatView statistical package (StatView 5, SAS Institute, Cary, North Carolina, USA) for statistical analyses. Nominal variables are provided as counts and percentages and compared by the two tailed Fischer exact test. Continuous variables, expressed as mean (SD), were compared by the Student t test. Multivariate logistic regression analysis was used to identify independent predictors of procedural events.
The analysed variables are reported with their respective odds ratios (OR) and 95% confidence intervals (CI).
RESULTS
Demographic and clinical characteristics of the patients
Demographic and clinical characteristics of the patients are given in table 1. The mean (SD) age of the population was 67 (8) years, with 26% of the patients aged between 70–74 years and 17% older than 75 years. Forty four per cent had contralateral carotid disease (36% with stenosis and 8% with carotid occlusion). Twelve per cent had undergone previous ipsilateral carotid endarterectomy, 16% previous contralateral carotid endarterectomy or angioplasty, 2% previous ipsilateral carotid stenting, and 1% previous subclavian–carotid bypass. In our cohort, 49 patients (49%) had coronary artery disease. Among these, seven with multivessel coronary disease were scheduled for coronary artery bypass grafting (CABG), nine had an acute coronary syndrome in the past six months, and 33 had previous myocardial infarction and either CABG or percutaneous transluminal coronary angioplasty (PTCA).
Table 1.
Baseline clinical characteristics of 100 patients with coronary artery stenting
| Sex | |
| Male | 71 |
| Female | 29 |
| Age (years) | |
| Range | 30–85 |
| Mean (SD) | 67 (8.2) |
| CAD (AP, AMI, CABG, PTCA) | 49 |
| Bilateral carotid disease | 44 |
| Contralateral occlusion | 8 |
| Diabetes | 17 |
| Hypertension | 78 |
| Hyperlipidaemia | 65 |
| Smoking: yes/no/prior | 11/50/40 |
| Ipsilateral previous PTACS | 2 |
| *Ipsilateral previous CEA or bypass | 13 |
| Contralateral previous PTACS/ previous CEA | 6/10 |
| Previous stroke/TIA | 28 |
Values are numbers of cases unless stated. As there were 100 patients in all, the numbers of cases are also percentages.
*12 patients with previous ipsilateral CEA and one with previous bypass LSA/LICA.
AMI, acute myocardial infarction; AP, angina pectoris; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CEA, carotid endarterectomy; LICA, left internal carotid artery; LSA, left subclavian artery ; PTACS, percutaneous transluminal angioplasty with carotid stenting; PTCA, percutaneous transluminal coronary angioplasty; TIA, transient ischaemic attack.
Twenty eight per cent of the patients suffered from previous cerebral ischaemic events. Accordingly to the Mayo Clinic carotid endarterectomy risk scale,4,19 73 patients were classified as grade III–IV and 13 as grade VI. The main risk associated variables used to stratify patients, and detailed descriptions of concomitant diseases present in each risk category, are reported in table 2.
Table 2.
Mayo Clinic carotid endarterectomy risk scale and characteristics of the 86 patients with Mayo class ≥III: main risk associated variables used to stratify patients and description of concomitant diseases present in each of the risk categories
| Mayo classification | |||
| Grade I | Low risk | ||
| Grade II | Angiographic risk | ||
| Grade III | Medical risk age ≥70, severe IHD, AP, or AMI ≤6 months, CHF, uncontrolled hypertension, severe peripheral occlusive vascular disease | ||
| Grade IV | High neurological risk | ||
| Grade V | Progressive neurological deficit within 6 hours | ||
| Grade VI | Previous ipsilateral CEA | ||
| Mayo class III (n=65) | Mayo class IV (n=8) | ||
| Age ≥70 years | 34 | Unstable neurological syndromes | 8 |
| Age alone | 15 | Bilateral carotid disease | 4 |
| Plus previous MI and/or PTCA | 8 | Contralateral carotid occlusion | 2 |
| Plus previous CABG | 9 | Mayo class VI (n=13) | |
| Plus CABG planned | 2 | Previous ipsilateral CEA | 12 |
| Significant CAD | 25 | Previous ipsilateral bypass | 1 |
| CABG planned | 5 | ||
| ACS <6 months | 9 | ||
| Previous CABG/MI and LVEF <40% | 5 | ||
| Previous PTCA and angina | 6 | ||
| PVD | 6 | ||
| Alone | 1 | ||
| Plus age ≥70 | 3 | ||
| Plus significant CAD | 2 | ||
ACS, acute coronary syndrome; AMI, acute myocardial infarction; AP, angina pectoris; CABG, coronary artery bypass graft; CAD, coronary artery disease; CEA, carotid endarterectomy; CHF, congestive heart failure; IHD, ischaemic hart disease; LVEF; left ventricular ejection fraction; PVD, peripheral vascular disease.
Procedural and angiographic characteristics
These data are given in table 3. Baseline lesion stenosis was 78.8 (10.6)% in the total population, 83.6 (8.5)% in symptomatic patients, and 77.5 (10.7)% in asymptomatic patients (NS). Mean lesion length was 12 (6) mm.
Table 3.
Lesion and procedural characteristics
| Segment | Baseline stenosis | TIMI flow | Final stenosis | Protection device | Stent† | |||||||||
| Vessel | Number of lesions (%) | Ostium | Proximal | Mid | Distal | % | Range | Pre | Post | %* | Range | Number of lesions | Yes | Yes |
| Right common carotid | 1(0.9) | 1 | 79.23 | 2 | 3 | 5.45 | 1 | 1 | 1 | |||||
| Left common carotid | 3 (2.9) | 2 (66) | 1 (33) | 79.1 (17) | 60–95.0 | 3 | 3 | 3.1 (5.0) | 0–9.2 | 3 | 1 | 2 | ||
| Right internal carotid | 45 (44.1) | 21 (46) | 23 (51) | 1 (2) | 80.3 (10) | 60–99 | 2–3 | 3 | 2.9 (4.0) | 0–10 | 43 | 33 | 42 | |
| Left internal carotid | 52 (50.9) | 22 (42) | 26 (25) | 4 (7.6) | 77.1 (10) | 60–95 | 3 | 3 | 1.9 (3.0) | 0–10 | 51 | 32 | 51 | |
| Bypass LSA/LICA anastomosis | 1 (0.9) | 81 | 3 | 3 | 0 | 1 | 0 | 1 | ||||||
| Total | 102 | n=45 (44%) | n=51 (50%) | n=5 (5%) | n=1 (1%) | 78.8 (10)% | 60–99 | 2.36 (3.5)% | 0–10 | n=99‡ (97%) | n=67 (67.6%) | n=97 (97.9%) | ||
Values are n or mean (SD) with range.
Lesion length: mean:12.63 (5.82) mm, range 2.4–34 mm.
Protection device: Neuroshield (28), Percusurge (17), Angioguard (14), Epi (8).
*The three lesions not treated are not included.
†Stent: Wallstent (74), X.Act (9), SMART (7), Expander (2), AVE (2), Corithian (1), Palmaz (2), Megalink (1), Dynalink (1).
‡Three carotids could not be cannulated owing to inability to approach the target vessel.
LICA, left internal carotid artery; LSA, left subclavian artery.
Angiographic success was obtained in 99 lesions (97%), with mean residual stenosis of 2.4 (3.5)%. Three lesions could not be treated because of inability to achieve selective catheterisation of the common carotid artery on account of severe tortuosity and extremely angulated take off. Of these three patients, one (who was symptomatic) was not considered eligible for carotid endarterectomy by the vascular surgeons because he had serious concomitant disease (severe coronary artery disease and peripheral vascular disease). He died one month later after an unrelated surgical procedure (leg amputation). The other two patients (both of whom were asymptomatic) refused to undergo vascular surgery and were event-free at the end of follow up.
Stenting was undertaken in 98% of the treated lesions; the remaining 2% were in-stent restenoses treated with balloon angioplasty alone. Direct stenting was done in 75% of the stented lesions, with balloon predilatation in the remaining 25%. Self expandable stents were used in 97% of the stented lesions and postdilatation was performed in all of these. Balloon expandable stents were used in 3% of the stented lesions.
We used four types of protection device in 67 lesions (67.6%): 28 Neuroshield (Mednova, Horsham, West Sussex, UK), 17 PercuSurge (Medtronic, Minneapolis, Minnesota, USA), 14 Angioguard (Cordis, Miami, Florida, USA), and eight Filterwire-Epi (Boston Scientific, Boston, Massachusetts, USA).
Predilatation was necessary to deliver the distal protection device in eight lesions. It was not associated with cerebral complications. Debris was present in 40% of the cases. Severe internal carotid vasospasm occurred in 4% and resolved promptly with intracarotid glyceryl trinitrate administration.
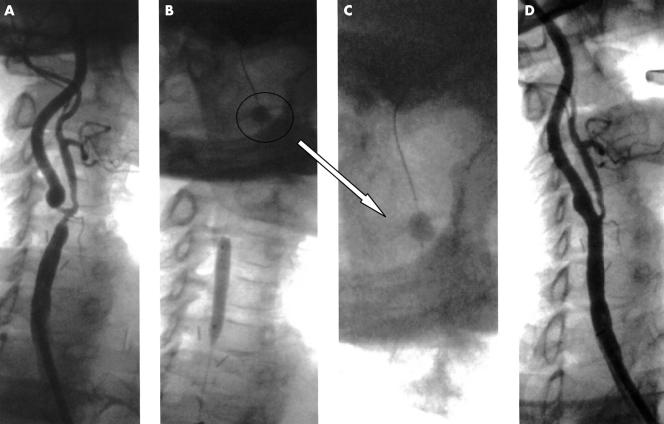
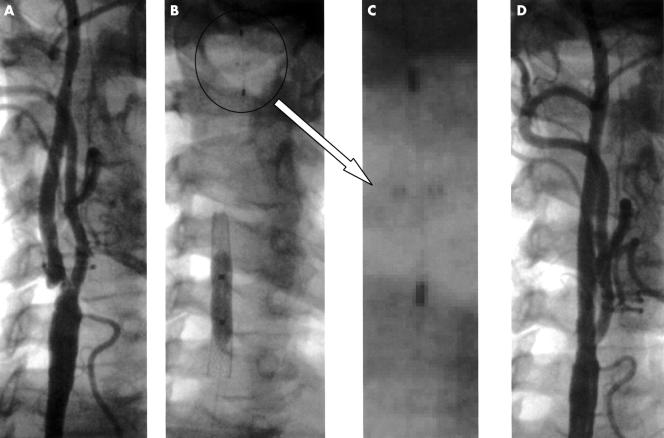
Angiographic findings from two patients pre- and postintervention are shown in figs 1 and 2.
Figure 1.
(A) Severe restenotic lesion at the right common carotid artery bifurcation after carotid endarterectomy, involving the ostia of both the internal and the external carotid. (B) In view of the lesion severity and tortuosity, the use of an Angioguard filter was excluded in favour of a PercuSurge GuardWire temporary occlusion and aspiration system (magnified in panel C). The system was positioned and the balloon was inflated without causing clinical symptoms. After predilatation with a coronary balloon 2.5 × 20 mm (panel B), an Easy Wallstent (Boston Scientific) was positioned between the proximal segment of the right internal carotid artery and the distal segment of the right common carotid artery. (D) After postdilatation with a 6.0 × 30 mm balloon at a pressure of 8 atm, final angiography showed an optimal result.
Figure 2.
(A) Angiography of the right carotid axis showing a significant lesion at the ostium of the internal carotid artery. (B) The lesion was suitable to use the Angioguard filter without predilatation. Once the filter was positioned and opened (magnified in panel C), direct stenting was undertaken with an Easy Wallstent 8.0 × 41 mm stent, postdilated with a 6.0 × 30 mm balloon at 6 atm (panel B). (D) Final angiography showed an optimal result. No debris was found in the filter once retrieved.
Procedural success was obtained in 96% of the patients. In-hospital events consisted of six periprocedural TIAs (6%) and one category 1 minor stroke (1%), which resolved completely after 28 hours. All patients left hospital without any neurological sequelae after an average stay of 2.5 days.
The following variables were entered into a logistic regression model: age (OR 0.95, 95% CI 0.86 to 1.05); concomitant coronary artery disease (OR 3.4, 95% CI 0.5 to 22.4); previous stroke/TIA (OR 1.0, 95% CI 0.1 to 7.4); bilateral carotid artery disease (OR 1.3, 95% CI 1.2 to 7.6); baseline lesion stenosis (OR 1.02, 95% CI 0.93 to 1.12); lesion length (OR 1.1, 95% CI 0.9 to 1.2); and the use of a protection device (OR 3.4, 95% CI 0.3 to 32.9); none was found to be a predictor of in-hospital events.
Follow up
During follow up (mean length 12 (6.2) months, range 3–26 months) six patients died. In two, death was related to a cerebrovascular accident (at 5 and 11 months after the procedure); in three it was related to acute cardiac events, and in one it occurred after unrelated surgery (leg amputation). No other acute neurological events were reported in the remaining population.
During follow up (table 4), 86 patients were evaluated by Doppler ultrasonography of the epiaortic vessels. Restenosis (per cent diameter stenosis > 50%) was found in three lesions (3.4%) at three, six, and 12 months. One patient with a carotid occlusion suffered from TIA and underwent carotid endarterectomy; another patient, who was asymptomatic, underwent a redo PTA; the remaining patient, who was also asymptomatic, was treated conservatively.
Table 4.
Follow up data
| Event | Number | Comment |
| Thrombosis | 0 | |
| Restenosis* | ||
| Moderate | 1 | Three months postprocedure the patient, who was asymptomatic, underwent re-PTA |
| Severe | 2 | One patient was asymptomatic and treated conservatively; the other developed carotid occlusion leading to TIA and underwent a successful CEA |
| TIA | 1 | |
| Minor stroke | 0 | |
| Major stroke | 0 | |
| Death | 6 | |
| Cerebral | 2 | At 5 and 11 month postprocedure |
| Non-cerebral | 4 | 3 cardiac cause and 1 during leg amputation |
| Clinical follow up only | 15 | All patients were asymptomatic |
Mean (SD) time of follow up: 12 (6.2) months, range 3–26 months.
*Detected by echo colour Doppler.
CEA, carotid endarterectomy; PTA, percutaneous transluminal angioplasty; TIA, transient ischaemic attack.
DISCUSSION
The aim of this paper was to report our experience with carotid stenting in our first 100 consecutive patients. The study population consisted of all the patients in whom carotid stenting was undertaken or intended. None of the referred patients was considered ineligible for carotid stenting for anatomical reasons or because of poor clinical condition. Nevertheless, in three patients the procedure could not be performed because of extreme tortuosity of the carotid system, which prevented selective cannulation of the artery. A previous study suggested that vessel tortuosity was a relative contraindication to carotid stenting.8
As observed in other reports of carotid artery stenting,7,8,13,20 there was a high incidence of concomitant coronary artery disease in our population. This is related to the fact that most of the patients were referred from other cardiologists, while those referred from vascular surgeons were at high risk for carotid endarterectomy because of the presence of severe coronary artery disease, impaired left ventricular function, or old age. Our study shows that carotid angioplasty with stent implantation may achieve excellent results, even in patients considered at high risk for carotid endarterectomy. Our complication rate, consisting of six patients with TIA and one with a category 1 minor stroke, is similar to the rates reported by other investigators,7,8,13,20 and may be better than those reported in carotid endarterectomy trials in patients with similar clinical characteristics.
Neither the presence of concomitant severe coronary artery disease nor the patient's age were predictors of in-hospital events. In the single case of minor stroke reported, clinical symptoms occurred two hours after the procedure, with complete resolution after approximately 28 hours. The possibility of late onset neurological complications after carotid stenting is reported by Wholey and colleagues.21 In our patient the event might have been caused by partial stent thrombosis or plaque fragmentation with resulting distal embolisation of small fragments or resistant spasm of the artery.
Although half of our patients had severe coronary disease, no periprocedural heart related complications were observed. The less invasive approach associated with percutaneous treatment, and the fact that it is performed under local anaesthesia, seems to limit cardiac complications in patients with combined carotid and coronary artery disease. It should be borne in mind that major adverse events (stroke, acute myocardial infarction, or death) have been reported in 8.8–10% of patients with severe coronary artery disease undergoing carotid endarterectomy.22 In the NASCET trial, cardiac complications occurred in 4.0% of the patients.23
A significant reduction in immediate complications has been reported when distal protection devices are used.12 In our cohort there was no difference in the incidence of in-hospital events depending on whether or not protection devices were used. The small number of patients treated and the very low rates of complications observed may explain this finding. Furthermore, all the procedural complications observed were uniformly distributed over the period in which the procedures were undertaken. This probably reflects the considerable technical experience of the operators, as reported in several studies. Carotid angioplasty and stenting are technically demanding procedures which should be performed only by well trained multidisciplinary teams.
The long term results in our population are similar to those reported by others.7,8,24–26 Though the statistical power of this small cohort does not allow definitive conclusions to be drawn, the relatively high mortality observed might be explained by the clinical characteristics of the population, with 49% of the patients suffering from coronary artery disease. In the two patients who died of stroke it was not possible to determine whether the event was ipsilateral or contralateral with respect to the lesion treated. As reported in previous studies,7,8,24,26 the restenosis rate in our population was very low and clinically silent in two of the three patients in whom it occurred. The patient with symptoms suffered from TIA, probably because of bilateral carotid artery disease and occlusive restenosis.
Conclusions
Carotid angioplasty with stent implantation seems to be a reliable and efficient method of treating occlusive carotid artery disease. In our experience, the risks do not seem greater than those of surgery, and might be lower in patients who are at high surgical risk. However, carotid angioplasty is technically demanding and should be performed only by well trained multidisciplinary teams using a clearly defined protocol for patient selection. Patient surveillance must be strict to detect any signs of restenosis or evidence of neurological injury. The results of several ongoing randomised studies comparing surgery and angioplasty will help to define further the role of stenting in the treatment of carotid occlusive disease.
Abbreviations
CABG, coronary artery bypass grafting
NASCET, North American symptomatic carotid endarterectomy trial
NIHSS, National Institutes of Health stroke scale
PTACS, percutaneous transluminal angioplasty with carotid artery stenting
TIA, transient ischaemic attack
REFERENCES
- 1.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American symptomatic carotid endarterectomy trial collaborators. N Engl J Med 1998;339:1415–25. [DOI] [PubMed] [Google Scholar]
- 2.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–8. [PubMed] [Google Scholar]
- 3.Winslow CM, Solomon DH, Chassin MR, et al. The appropriateness of carotid endarterectomy. N Engl J Med. 1988;318:721–7. [DOI] [PubMed] [Google Scholar]
- 4.Sundt TM, Meyer FB, Piepgras DG, et al. Risk factors and operative results. In: Meyer FB, ed. Sundt's occlusive cerebrovascular disease, 2nd ed. Philadelphia: WB Saunders Co, 1994:241–7.
- 5.MCR European Carotid Surgery Trial Investigators. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MCR European carotid surgery trial (ECST). Lancet 1998;351:1379–87. [PubMed] [Google Scholar]
- 6.Kerber CW, Cromwell LD, Loehden OL. Catheter dilatation of proximal carotid stenosis during distal bifurcation endarterectomy. Am J Neuroradiol 1980;1:348–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Yadov JS, Roubin GS, Iyer S, et al. Elective stenting of extracranial carotid arteries. Circulation 1997;95:376–81. [DOI] [PubMed] [Google Scholar]
- 8.Henry M, Amor M, Henry I, et al. Angioplasty and stenting of the external carotid arteries. J Endovasc Surg 1998;5:293–304. [DOI] [PubMed] [Google Scholar]
- 9.Wholey MH, Bergeron P, Diethrich E, et al. Current global status of carotid artery stent placement. Cathet Cardiovasc Diagn 1998;44:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mubarak N, Roubin GS, Ming W, et al. Early results of percutaneous intervention for severe coexisting carotid and coronary artery disease. Am J Cardiol 1999;84:600–2. [DOI] [PubMed] [Google Scholar]
- 11.Al-Mubarak N, Roubin GS, Gomez CR, et al. Carotid artery stenting in patients with high neurologic risks. Am J Cardiol 1999;83:1411–13. [DOI] [PubMed] [Google Scholar]
- 12.Reimers B, Corvaja N, Moshiri S, et al. Cerebral protection with filter device during carotid artery stenting. Circulation 2001;104:12–15. [DOI] [PubMed] [Google Scholar]
- 13.Shawl F, Kadro W, Domanski MJ, et al. Safety and efficacy of elective carotid artery stenting in high risk patients. J Am Coll Cardiol 2000;35:1721–8. [DOI] [PubMed] [Google Scholar]
- 14.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the trial of Org 10172 in acute stroke treatment (TOAST). Neurology 1999. 13;53:126–31. [DOI] [PubMed] [Google Scholar]
- 16.Neschis DG, Lexa FJ, Davis JT, et al. Duplex criteria for determination of 50% or greater carotid stenosis. Br J Surg 2001;20:207–15. [DOI] [PubMed] [Google Scholar]
- 17.Gomez CR, Roubin GS, Dean LS, et al. Neurological monitoring during carotid artery stenting: the Duke squeezing test. J Endovasc Surg 1999;6:332–6. [DOI] [PubMed] [Google Scholar]
- 18.Gagne PJ, Matchett J, MacFarland D, et al. Can the NASCET technique for measuring carotid stenosis be reliably applied outside the trial? J Vasc Surg 1996;24:449–56. [DOI] [PubMed] [Google Scholar]
- 19.Sundt TM, Sandok BA, Whisuant JP. Carotid endarterectomy: complications and pre-operative assessment of risk. Mayo Clin Proc 1975;50:301–6. [PubMed] [Google Scholar]
- 20.Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239–45. [DOI] [PubMed] [Google Scholar]
- 21.Wholey MH, Tan WA, Toursarkissian B, et al. Management of neurological complications of carotid artery stenting. J Endovasc Ther 2001;8:341–53. [DOI] [PubMed] [Google Scholar]
- 22.Al-Mubarak N, Roubin GS, Liu MW, et al. Early results of percutaneous intervention for severe coexisting carotid and coronary artery disease [abstract]. Am J Cardiol 1999;84:600–2, A9. [DOI] [PubMed] [Google Scholar]
- 23.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53. [DOI] [PubMed] [Google Scholar]
- 24.Roubin GS, New G, Iyer S, et al. Immediate and late clinical outcome of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-years prospective analysis. Circulation 2001;103:532–7. [DOI] [PubMed] [Google Scholar]
- 25.Mathias K, Jager MJ, Sahl H, et al. Interventional treatment of arteriosclerotic carotid stenosis. Radiology 1999;39:125–34. [DOI] [PubMed] [Google Scholar]
- 26.Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid stent placement. Cathet Cardiovasc Intervent 2000;50:160–7. [DOI] [PubMed] [Google Scholar]