Abstract
Objective: To describe the clinical features, management, and prognosis of patients presenting with clinical markers of spontaneous reperfusion (SR) during acute myocardial infarction (AMI).
Design: Cohort study.
Setting: National registry of 26 coronary care units.
Patients: 2382 consecutive patients with AMI.
Main outcome measures: Patient characteristics, management, and mortality.
Results: The incidence of SR was 4% of patients (n = 98) compared with thrombolytic treatment (n = 1163, 49%), primary angioplasty (n = 102, 4%), and non-reperfusion (n = 1019, 43%). SR patients were more likely to develop less or no myocardial damage as indicated by a higher percentage of non-Q wave AMI (58% v 32%, 47%, and 44%, respectively, p < 0.0001), aborted AMI (25% v 9%, 8%, and 12%, p < 0.001), and lower peak creatine kinase (503 v 1384, 1519, and 751 IU, p < 0.0001). SR patients, however, were more likely to develop recurrent ischaemic events (35% v 17%, 12%, and 16%, respectively; p < 0.001) and subsequently were more likely to be referred to coronary angiography (67%), angioplasty (41%), or bypass surgery (16%, p < 0.001). Mortality at 30 days (1% v 8%, 7%, and 13%, respectively, p < 0.0001) and one year (6% v 11%, 12%, and 19%, p < 0.0001) was significantly lower for SR patients than for the other subgroups. By multivariate analysis, SR remained a strong determinant of 30 day survival (odds ratio (OR) 0.16, 95% confidence interval (CI) 0.01 to 0.74). At one year, the association between SR and survival decreased (OR 0.49, 95% CI 0.18 to 1.13).
Conclusions: Clinical markers of SR are associated with greater myocardial salvage and favourable prognosis. The vulnerability of SR patients to recurrent ischaemic events suggests that they need close surveillance and may benefit from early intervention.
Keywords: acute myocardial infarction, angina, reperfusion, thrombolysis
The significance and implications of clinical markers of spontaneous reperfusion (SR) in acute myocardial infarction (AMI) have not yet been investigated in detail. The incidence of SR during acute AMI, reported by angiographic studies, varies widely (7–57%).1–6 Recent studies showed that “spontaneous” reperfusion before primary percutaneous transluminal coronary angioplasty (PTCA) is an independent determinant of procedural success, myocardial salvage, and improved outcome.4–6 However, data on characteristics, management, and outcome of patients with AMI presenting with clinical markers of SR and who are treated conservatively are limited.
We therefore decided to evaluate patients who presented with clinical markers of SR during AMI as compared with AMI patients who did not. Better characterisation and additional knowledge of SR may promote early recognition and risk stratification, avoid unnecessary procedures and complications, and improve outcome.
PATIENTS AND METHODS
Patients admitted to all 26 coronary care units in Israel were prospectively enrolled in a national survey, conducted in January through February and May through July 1996.7 Briefly, patient demographic data, medical history, hospital management, hospital complications, and 30 day and 12 month follow up were prospectively recorded on a predefined survey form. The reasons for excluding patients from thrombolytic treatment, including SR, were recorded. Medical records of all patients with SR were retrospectively reviewed by two investigators to validate the diagnosis of SR. All surviving patients were seen or contacted by telephone at 30 days. One year mortality data were obtained from records of the Ministry of Interior.
Definitions
Diagnosis of AMI was based on the presence of any two of the following criteria:
typical chest pain lasting 30 minutes or longer
unequivocal new ECG changes (Q/QS or ST segment deviation or peaked, tall T waves or T wave inversion)
an increase in creatine kinase (CK) to more than twice the upper normal limit of each hospital laboratory and a concomitant increase in CK MB isoenzyme.
For the purpose of our study, SR was defined by clinical criteria8–11 in patients with AMI who were fully eligible for thrombolysis but did not receive reperfusion because they developed, within six hours from symptom onset, markers of SR, defined as follows:
spontaneous, complete, or partial (> 50%) resolution of ST segment elevation as diagnosed by serial (at least two) ECGs that were obtained before hospital admission or at the emergency department and the coronary care unit
significant relief of chest pain
early inversion of T waves in the infarct related ECG leads
accelerated idioventricular rhythm.
The first two criteria were essential for the diagnosis of SR. The criteria were selected following a review of the literature, with emphasis on criteria that could be measured quickly and at the bedside.
Aborted AMI12 was defined as AMI that, because of SR, thrombolysis, or primary PTCA, resulted in peak CK < 250 IU and absence of the Q wave in the discharge ECG.
Statistical analysis
Statistical analysis was performed using SAS software (version 6, SAS Institute, Cary, North Carolina, USA). Continuous variables are expressed as mean (1 SD), or as medians with 25th and 75th centiles where appropriate. Differences between SR and other subgroups were calculated by analysis of variance or the Kruskal-Wallis test (non-parametric analysis of variance) where appropriate. Differences between SR and thrombolysis treated patients were calculated by the t test or Wilcoxon test, where appropriate. Differences between categorical variables were analysed by the χ2 test. A probability value of p < 0.05 was considered significant. All p values are two tailed.
We performed logistic regression analyses to assess the independent association of various variables with mortality using the SAS LOGISTIC procedure. Variables included in the model were age, sex, history of AMI, congestive heart failure, smoking, angina 48 hours before admission, hypertension, diabetes mellitus, anterior AMI, time from symptom onset to admission (< 2 hours), in-hospital use of nitrates or angiotensin converting enzyme (ACE) inhibitors, and PTCA or coronary artery bypass grafting (CABG) in hospital.
To determine whether there is an association between certain clinical variables or medications and the occurrence of SR, we performed stepwise logistic regression analysis on SR and thrombolysis treated patients. The first model included age, sex, past myocardial infarction, premyocardial infarction angina, smoking, hypertension, anterior myocardial infarction, Q wave myocardial infarction, congestive heart failure, and diabetes mellitus. The second model included the above mentioned variables, with the addition of prior (seven days) use of ACE inhibitors, nitrates, aspirin, insulin, and calcium channel blockers.
RESULTS
Of the 2382 AMI patients, 98 patients (4%) met our clinical criteria of SR. Thrombolytic treatment was administered to 1163 (49%) patients and 102 (4%) patients were treated with primary PTCA. Other patients (n = 1019, 43%) were not treated with acute reperfusion.
Baseline characteristics
Table 1 compares the clinical characteristics of the patient subgroups. SR patients were slightly older than thrombolysis or primary PTCA treated patients, had a higher prevalence of hypertension, and were more likely to experience angina in the 48 hours before infarction (table 1). SR patients arrived at the hospital later than patients treated with reperfusion but earlier than non-reperfused patients. For thrombolysis treated patients, the door to needle time (median (25th to 75th centiles)) was 71 (49–106) minutes. Major reasons for exclusion from thrombolysis were late arrival (n = 342, 34%), lack of ECG criteria (n = 303, 30%), and contraindications to thrombolysis (n = 210, 21%). SR patients were more likely to have a better haemodynamic status as reflected by the higher proportion of patients in Killip class 1 on admission and lower heart rate. Patients selected for primary PTCA were at high risk as reflected by the higher percentage of anterior myocardial infarction and heart failure on admission.
Table 1.
Baseline characteristics
| Spontaneous reperfusion (n=98) | Thrombolysis (n=1163) | Primary PTCA (n=102) | No reperfusion (n=1019) | p Value | |
| Age (years) (mean (SD)) | 63 (12) | 61 (12) | 60 (12) | 65 (13) | <0.0001 |
| Women | 29 (30%) | 263 (23%) | 19 (19%) | 297 (29%) | 0.001 |
| Medical history | |||||
| Myocardial infarction | 18 (18%) | 186 (16%) | 31 (30%) | 300 (29%) | <0.0001 |
| Diabetes mellitus | 21 (21%) | 272 (23%) | 28 (27%) | 272 (27%) | 0.25 |
| Current smoking | 48 (49%) | 341 (43%) | 49 (48%) | 273 (27%) | <0.0001 |
| Hypertension | 44 (45%) | 405 (35%) | 34 (33%) | 493 (48%) | <0.0001 |
| Dyslipidaemia | 36 (37%) | 336 (29%) | 30 (29%) | 289 (28%) | 0.38 |
| Prior angina | 38(39%) | 436 (37%) | 39 (38%) | 411 (40%) | 0.60 |
| Angina 48 h before admission | 15 (15%) | 110 (9 %) | 10 (10%) | 89 (9%) | 0.21 |
| Previous medications | |||||
| β Blockers | 16 (16%) | 155 (13%) | 21 (21%) | 164 (16%) | 0.10 |
| Nitrates | 12 (12%) | 263 (23%) | 19 (19%) | 233 (23%) | 0.08 |
| Calcium channel blockers | 19 (19%) | 215 (19%) | 20 (20%) | 217 (21%) | 0.44 |
| Aspirin | 27 (28%) | 283 (24%) | 39 (38%) | 277 (27%) | 0.01 |
| ACE inhibitors | 16 (16%) | 117 (10%) | 10 (10%) | 175 (17%) | 0.0001 |
| Time (minutes) from symptom onset to emergency room admission (median (25th to 75th centiles)) | 136 (73–266) | 103 (60–175) | 108 (61–188) | 220 (105–516) | <0.0001 |
| Anterior AMI | 48 (50%) | 526 (47%) | 65 (64%) | 426 (45%) | 0.001 |
| Q wave AMI | 33 (37%) | 694 (65%) | 50 (53%) | 497 (51%) | 0.001 |
| Non-Q wave AMI | 56 (57%) | 373 (32%) | 44 (47%) | 422 (44%) | 0.001 |
| Aborted AMI* (CK <250 IU) | 23 (25%) | 73 (6%) | 7 (8%) | 113 (12%) | 0.001 |
| CK (IU) (median (25th to 75th centiles) | 503 (254–818) | 1384 (685–2599) | 1519 (774–3297) | 751 (420–1341) | <0.0001 |
| Heart failure on admission (Killip >1) | 7 (7%) | 187 (16%) | 37 (36%) | 282 (28%) | 0.001 |
| Admission heart rate (beats/min) (mean (SD)) | 75 (14) | 78 (18) | 86 (22) | 81 (20) | <0.0001 |
| Admission systolic blood pressure (mm Hg) (mean (SD)) | 138 (27) | 136 (24) | 128 (29) | 135 (27) | 0.02 |
*Missing data on creatine kinase (CK) in 4, 26, 10, and 53 patients in each group, respectively. ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Previous medical treatment during the seven days before infarction differed between SR and reperfusion treated patients with regard to two drugs—SR patients were more likely to have been taking ACE inhibitors but less likely to receive nitrates. There was no difference in the frequency of previous use of aspirin, calcium channel blockers, or β blockers (table 1).
We observed a lesser extent of myocardial damage in SR patients (table 1), as indicated by a higher incidence of aborted AMI (CK < 250 IU), a less frequent evolution of Q wave AMI, and a lower peak CK concentration (table 1).
In-hospital course
Compared with other AMI patients, patients with SR were more likely to develop recurrent ischaemia during hospitalisation. Compared with thrombolysis treated patients, there was no difference in the incidence of other complications such as heart failure or shock (table 2).
Table 2.
In-hospital complications, treatment, and procedures
| Spontaneous reperfusion (n=98) | Thrombolysis (n=1163) | Primary PTCA (n=102) | No reperfusion (n=1017) | p Value | |
| Recurrent ischaemia | 34 (35%) | 196 (17%) | 12 (12%) | 161 (16%) | <0.001 |
| Heart failure | 12 (12%) | 151 (13%) | 21 (21%) | 197 (19%) | <0.001 |
| Cardiogenic shock | 1 (1%) | 33 (3%) | 14 (14%) | 69 (7%) | <0.0001 |
| VT or VF | 10 (10%) | 174 (15%) | 31 (30%) | 112 (11%) | <0.0001 |
| Recurrent AMI | 3 (3%) | 37 (3%) | 2 (2%) | 18 (2%) | 0.2 |
| In-hospital treatment | |||||
| Aspirin | 90 (92%) | 1100 (95%) | 99 (97%) | 869 (85%) | <0.0001 |
| β Blockers | 58 (59%) | 703 (60%) | 44 (43%) | 512 (50%) | <0.0001 |
| ACE inhibitors | 42 (43%) | 602 (52%) | 62 (61%) | 492 (48%) | 0.02 |
| Heparin | 89 (91%) | 970 (83%) | 90 (88%) | 814 (80%) | 0.006 |
| In-hospital procedures | |||||
| Coronary angiography | 51 (52%) | 434 (37%) | 102 (100%) | 305 (30%) | <0.0001 |
| PTCA | 32 (33%) | 244 (21%) | 102 (100%) | 140 (14%) | <0.0001 |
| CABG | 11 (11%) | 61 (5%) | 3 (3%) | 55 (5%) | 0.05 |
| Procedures within 30 days | |||||
| Coronary angiography | 66 (67%) | 585 (50%) | 102 (100%) | 441 (43%) | <0.0001 |
| PTCA | 40 (41%) | 303 (26%) | 102 (100%) | 180 (18%) | <0.0001 |
| CABG | 16 (16%) | 105 (9%) | 6 (6%) | 106 (10%) | 0.05 |
ACE, angiotensin converting enzyme; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty; VF, ventricular fibrillation; VT, ventricular tachycardia.
Compared with thrombolysis treated and non-reperfused patients, SR patients were more likely to undergo coronary angiography, PTCA, or CABG during hospitalisation or within 30 days (table 2).
Mortality
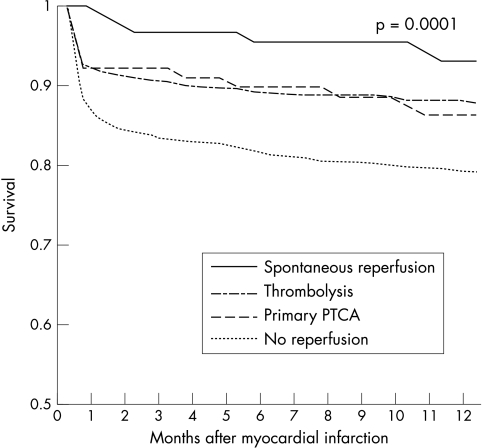
Mortality of patients with SR was lower than that of reperfusion treated and non-reperfused patients at seven days, at one month, and at one year (table 3, fig 1).
Table 3.
Mortality after myocardial infarction
| Mortality | Spontaneous reperfusion (n=98) | Thrombolysis (n=1163) | Primary PTCA (n=102) | No reperfusion (n=1019) | p Value |
| 7 days | 0 (0%) | 66 (6%) | 4 (4%) | 83 (8%) | 0.003 |
| 30 days | 1 (1%) | 91 (8%) | 7 (7%) | 132 (13%) | <0.0001 |
| 1 year | 6 (6%) | 130 (11%) | 12 (12%) | 191 (19%) | <0.0001 |
| 30 day OR* (95% CI) | 0.16 (0.01 to 0.74) | 0.94 (0.67 to 1.32) | 1.12 (0.41 to 2.68) | 1.0 | |
| 1 year OR* (95% CI) | 0.49 (0.18 to 1.13) | 0.94 (0.71 to 1.26) | 1.12 (0.52 to 2.30) | 1.0 |
*Adjusted for age, sex, history of AMI, congestive heart failure, smoking, angina 48 hours before admission, hypertension, diabetes mellitus, anterior AMI, time from symptom onset to admission (<2 hours), in hospital use of nitrates or ACE inhibitors, and PTCA or CABG in hospital.
CI, confidence interval; OR, odds ratio.
Figure 1.
Kaplan-Meier survival curve. Cumulative survival after acute myocardial infarction in patients according to reperfusion treatment (plog rank test = 0.0001).
After covariate adjustment (table 3), with the non-reperfused group as the reference group, SR was independently associated with improved survival at 30 days (odds ratio (OR) 0.16, 95% confidence interval (CI) 0.01 to 0.74). At one year the association between SR and survival decreased (OR 0.49, 95% CI 0.18 to 1.13).
Variables associated with the occurrence of SR
In attempting to identify variables associated with the evolution of SR we focused on a subgroup of patients eligible for thrombolysis as a reference group. Patients who were referred for primary PTCA constituted a selected, high risk group and non-reperfused patients arrived in a significant delay. Compared with thrombolysis treated patients, among SR patients several clinical variables and medication use were more frequent (table 1). Age, preinfarction angina, hypertension, non-Q wave myocardial infarction, and Killip class 1 on admission emerged as independent variables associated with the occurrence of SR (table 4). After the use of previous medications was added into the model, previous treatment with nitrates was shown to have an inverse association with SR (table 4). The interaction between SR and time to admission (> 2 hours) was examined and found not to be significant.
Table 4.
Variables associated with the evolution of spontaneous reperfusion
| Odds ratio | 95% CI | |
| A. Logistic model without previous medications | ||
| Age (for 1 year increment) | 1.02 | 1.00 to 1.04 |
| Preinfarction angina (<48 h) | 1.80 | 0.95 to 3.22 |
| Hypertension | 1.50 | 0.94 to 2.38 |
| Q wave myocardial infarction | 0.32 | 0.20 to 0.51 |
| Heart failure on admission (Killip class >1) | 0.33 | 0.12 to 0.73 |
| B. Logistic model with previous medications | ||
| Age (for 1 year increment) | 1.02 | 1.00 to 1.04 |
| Preinfarction angina (<48 h) | 1.76 | 0.92 to 3.19 |
| Hypertension | 1.53 | 0.89 to 2.58 |
| Q wave myocardial infarction | 0.34 | 0.21 to 0.53 |
| Heart failure on admission (Killip class >1) | 0.31 | 0.11 to 0.69 |
| Previous medications | ||
| Aspirin | 1.50 | 0.84 to 2.61 |
| ACE inhibitors | 1.60 | 0.76 to 3.17 |
| Nitrates | 0.31 | 0.13 to 0.64 |
DISCUSSION
The major findings of our study are as follows. Firstly, patients with clinical markers of SR are more likely to develop less or no myocardial damage than reperfusion treated or non-reperfused patients. Secondly, these patients are predisposed to recurrent ischaemic events and consequently are more likely to be referred for cardiac catheterisation, PTCA, or CABG. Thirdly, clinical SR is associated with improved survival compared with patients treated with thrombolysis or primary PTCA. Our combined observations support the data indicating the benefits of early reperfusion in AMI.
Comparison with previous studies
Most of the recent reports on the occurrence of SR have been based on data from AMI patients who were referred for primary PTCA.4–6 This information showed that patients with SR tended to have all the surrogates of improved myocardial salvage3: less cardiac enzyme release, a higher left ventricular ejection fraction, fewer complications, and a better outcome than patients in whom reperfusion was required to achieve TIMI (thrombolysis in myocardial infarction) grade 3 patency. Furthermore, SR in AMI was associated with faster coronary flow after primary angioplasty.5
A recent analysis6 of > 2500 patients in the four PAMI (primary angioplasty in myocardial infarction) trials compared patients who achieved TIMI grade 3 flow “spontaneously” on the angiography before primary PTCA (16% of the population) with those who had TIMI 0 to 2 flow.6 Those with spontaneous TIMI grade 3 flow had improved left ventricular function, a lower rate of congestive heart failure, and lower mortality. In addition, the authors observed that procedural success was higher in patients with baseline TIMI 3 flow.
Our and other studies1,13 found a higher rate of recurrent ischaemic events among SR patients. A possible explanation is that greater myocardial salvage, without complete revascularisation, renders more viable myocardium vulnerable to subsequent ischaemia and infarction.14,15 The higher incidence of reischaemia among SR patients may explain the higher use of coronary angiography, PTCA, and CABG in this subgroup.
Determinants of SR
We identified preinfarction angina and hypertension as clinical variables associated with the occurrence of SR. Q wave AMI and heart failure on admission had inverse associations with SR. Recent clinical reports suggest that preinfarct angina is associated with SR5 or more rapid thrombolysis.16 In experimental studies, brief “preconditioning” ischaemia, in addition to its ability to render myocytes resistant to infarction, may also have favourable effects on arterial patency.17,18 Release of adenosine from ischaemic/reperfused myocardium and resultant adenosine receptor stimulation may contribute to enhanced coronary patency.17
We observed a borderline association between previous ACE inhibitor or aspirin treatment and the occurrence of SR. This is likely to be mediated by the beneficial effects of treatment on vascular reactivity and the coagulation system.19,20 ACE inhibitors reduce recurrent ischaemic events21,22 and restore sympathovagal imbalance. ACE inhibitors may also improve endothelial function and counteract reduced fibrinolysis by suppression of plasminogen activator inhibitor 1 expression.19
The provocative and new observation of an inverse association between previous nitrate treatment and SR is surprising. The mechanisms for this can only be speculated on; recent evidence suggests that continuous treatment with nitrates may worsen endothelial function in the coronary arteries of patients with coronary artery disease, including increased superoxide anion or endothelin production and sensitivity to vasoconstrictors.23,24 This mechanism appears to have an important role in the development of nitrate tolerance.23,24 Recent analysis has suggested that continuous treatment with nitrates may worsen the prognosis of patients with ischaemic heart disease.25,26 In our study, however, it is possible that nitrates simply were a surrogate for more severe cardiac disease.
Limitations
Our analysis was done retrospectively and the available data depend on the quality of the information recorded by the research nurses and physicians. To overcome this limitation and to confirm the diagnosis of SR, we reviewed medical records of all patients with SR. We are aware of the weakness that we did not review the records of non-reperfused patients.
Compared with previous reports, the 4% incidence of SR is relatively low.1–6 Possible explanations are, firstly, that our clinical criteria8,9 are less sensitive than coronary angiography in identifying coronary artery patency. Secondly, the patency of the infarct related artery may increase with time.2,27 We studied patients with SR < 6 hours while several studies included patients up to 24 hours after symptom onset. Thirdly, other studies included selected candidates for primary PTCA1,4–6 who were treated with aspirin, heparin, and ticlopidine, which may promote the evolution of SR. Finally, our ECG criteria may reflect myocardial reperfusion and are less sensitive in detecting coronary reperfusion.11,28,29 On the other hand, using bedside criteria for myocardial reperfusion is more convenient and has strong prognostic implications.11,28,29
The longer time from symptom onset to emergency room admission in SR patients may introduce a form of selection bias in that these patients have already survived longer than reperfusion treated patients. However, the interaction between SR and time to admission (> 2 hours) was examined and found not to be significant.
Conclusions and implications
Our study has shown that bedside clinical markers of SR are associated with smaller infarcts and more favourable outcome than in AMI patients treated with thrombolysis or primary PTCA. SR patients, however, are prone to recurrent ischaemic events. The implication of our study is that SR patients need close surveillance and early risk stratification. They may be protected by strategies to prevent reischaemia such as use of aspirin, heparin, β receptor blockers, ACE inhibitors, statins, and clopidogrel. These patients may benefit from early coronary angiography and coronary artery revascularisation. The best time for coronary intervention, immediate versus delayed, needs further investigation.
Acknowledgments
The first two authors contributed equally. This study was done as part of the MD thesis requirements at the Faculty of Health Sciences, Ben-Gurion University.
Abbreviations
ACE, angiotensin converting enzyme
AMI, acute myocardial infarction
CABG, coronary artery bypass grafting
CK, creatine kinase
PAMI, primary angioplasty in myocardial infarction
PTCA, percutaneous coronary angioplasty
SR, spontaneous reperfusion
TIMI, thrombolysis in myocardial infarction
REFERENCES
- 1.Steg PG, Himbert D, Benamer H, et al. Conservative management of patients with acute myocardial infarction and spontaneous acute patency of the infarct-related artery. Am Heart J 1997;134:248–52. [DOI] [PubMed] [Google Scholar]
- 2.DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897–902. [DOI] [PubMed] [Google Scholar]
- 3.Christian TF, Milavetz JJ, Miller TD, et al. Prevalence of spontaneous reperfusion and associated myocardial salvage in patients with acute myocardial infarction. Am Heart J 1998;135:421–7. [DOI] [PubMed] [Google Scholar]
- 4.Anon. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. The global use of strategies to open occluded coronary arteries in acute coronary syndromes (GUSTO IIb) angioplasty substudy investigators. N Engl J Med 1997;336:1621–8. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Hong MK, Lee JH, et al. Determinants and prognostic significance of spontaneous coronary recanalization in acute myocardial infarction. Am J Cardiol 2001;87:951–4, A953.. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation 2001;104:636–41. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb S, Harpaz D, Shotan A, et al. Sex differences in management and outcome after acute myocardial infarction in the 1990s: a prospective observational community-based study. Israeli thrombolytic survey group. Circulation 2000;102:2484–90. [DOI] [PubMed] [Google Scholar]
- 8.Oldroyd KG. Identifying failure to achieve complete (TIMI 3) reperfusion following thrombolytic treatment: how to do it, when to do it, and why it’s worth doing. Heart 2000;84:113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaturi M, Birnbaum Y. The use of the electrocardiogram to identify epicardial coronary and tissue reperfusion in acute myocardial infarction. J Thromb Thrombolysis 2000;10:137–47. [DOI] [PubMed] [Google Scholar]
- 10.Matetzky S, Barabash GI, Shahar A, et al. Early T wave inversion after thrombolytic therapy predicts better coronary perfusion: clinical and angiographic study. J Am Coll Cardiol 1994;24:378–83. [DOI] [PubMed] [Google Scholar]
- 11.Matetzky S, Freimark D, Chouraqui P, et al. The distinction between coronary and myocardial reperfusion after thrombolytic therapy by clinical markers of reperfusion. J Am Coll Cardiol 1998;32:1326–30. [DOI] [PubMed] [Google Scholar]
- 12.Lamfers EJ, Hooghoudt TE, Uppelschoten A, et al. Effect of prehospital thrombolysis on aborting acute myocardial infarction. Am J Cardiol 1999;84:928–30, A926–7.. [DOI] [PubMed] [Google Scholar]
- 13.Huey BL, Gheorghiade M, Crampton RS, et al. Acute non-Q wave myocardial infarction associated with early ST segment elevation: evidence for spontaneous coronary reperfusion and implications for thrombolytic trials. J Am Coll Cardiol 1987;9:18–25. [DOI] [PubMed] [Google Scholar]
- 14.Gersh BJ, Anderson JL. Thrombolysis and myocardial salvage: results of clinical trials and the animal paradigm: paradoxic or predictable? Circulation 1993;88:296–306. [DOI] [PubMed] [Google Scholar]
- 15.Chareonthaitawee P, Gibbons RJ, Roberts RS, et al. The impact of time to thrombolytic treatment on outcome in patients with acute myocardial infarction. For the CORE investigators (collaborative organisation for RheothRx evaluation). Heart 2000;84:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreotti F, Pasceri V, Hackett DR, et al. Preinfarction angina as a predictor of more rapid coronary thrombolysis in patients with acute myocardial infarction. N Engl J Med 1996;334:7–12. [DOI] [PubMed] [Google Scholar]
- 17.Hata K, Whittaker P, Kloner RA, et al. Brief antecedent ischemia attenuates platelet-mediated thrombosis in damaged and stenotic canine coronary arteries: role of adenosine. Circulation 1998;97:692–702. [DOI] [PubMed] [Google Scholar]
- 18.Hata K, Whittaker P, Kloner RA, et al. Brief myocardial ischemia attenuates platelet thrombosis in remote, damaged, and stenotic carotid arteries. Circulation 1999;100:843–8. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan DE. Angiotensin, fibrinolysis, and vascular homeostasis. Am J Cardiol 2001;87:18C–24C. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan DE, Rouleau JL, Ridker PM, et al. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation 1997;96:442–7. [DOI] [PubMed] [Google Scholar]
- 21.Kennon S, Barakat K, Hitman GA, et al. Angiotensin-converting enzyme inhibition is associated with reduced troponin release in non-ST-elevation acute coronary syndromes. J Am Coll Cardiol 2001;38:724–8. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- 23.Gori T, Mak SS, Kelly S, et al. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J Am Coll Cardiol 2001;38:1096–101. [DOI] [PubMed] [Google Scholar]
- 24.Munzel T. Does nitroglycerin therapy hit the endothelium? J Am Coll Cardiol 2001;38:1102–5. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Moss AJ, Brown MW, et al. Long-term nitrate use may be deleterious in ischemic heart disease: a study using the databases from two large-scale postinfarction studies. Multicenter myocardial ischemia research group. Am Heart J 1999;138:577–85. [DOI] [PubMed] [Google Scholar]
- 26.Teo KK, Catellier DJ. Long-term nitrate use in chronic coronary artery disease: need for a randomized controlled trial. Am Heart J 1999;138:400–2. [DOI] [PubMed] [Google Scholar]
- 27.Rentrop KP. Thrombi in acute coronary syndromes : revisited and revised. Circulation 2000;101:1619–26. [DOI] [PubMed] [Google Scholar]
- 28.Kaul S. Coronary angiography cannot be used to assess myocardial perfusion in patients undergoing reperfusion for acute myocardial infarction. Heart 2001;86:483–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol 2001;38:1283–94. [DOI] [PubMed] [Google Scholar]