Abstract
Objective: To re-examine the standard pNN50 heart rate variability (HRV) statistic by determining how other thresholds compare with the commonly adopted 50 ms threshold in distinguishing physiological and pathological groups.
Design: Retrospective analysis of Holter monitor databases.
Subjects: Comparison of HRV data between 72 healthy subjects and 43 with congestive heart failure (CHF); between sleeping and waking states in the 72 healthy subjects; and between 20 young and 20 healthy elderly subjects.
Main outcome measures: Probability values for discriminating between groups using a family of pNN values ranging from pNN4 to pNN100.
Results: For all three comparisons, pNN values substantially less than 50 ms consistently provided better separation between groups. For the normal versus CHF groups, p < 10−13 for pNN12 versus p < 10−4 for pNN50; for the sleeping versus awake groups, p < 10−21 for pNN12 versus p < 10−10 for pNN50; and for the young versus elderly groups, p < 10−6 for pNN28 versus p < 10−4 for pNN50. In addition, for the subgroups of elderly healthy subjects versus younger patients with CHF, p < 0.007 for pNN20 versus p < 0.17 for pNN50; and for the subgroup of New York Heart Association functional class I–II CHF versus class III–IV, p < 0.04 for pNN10 versus p < 0.13 for pNN50.
Conclusions: pNN50 is only one member of a general pNNx family of HRV statistics. Enhanced discrimination between a variety of normal and pathological conditions is obtained by using pNN thresholds as low as 20 ms or less rather than the standard 50 ms threshold.
Keywords: aging, autonomic nervous system, congestive heart failure, pNN50
pNN50 is a widely used measure of heart rate variability (HRV). This statistic is derived from the 1984 study of Ewing and colleagues,1 which introduced the NN50 count, defined as the mean number of times an hour in which the change in successive normal sinus (NN) intervals exceeds 50 ms. The authors proposed this measure to help assess parasympathetic activity from 24 hour ECG recordings and presented supporting data from healthy subjects compared with those with diabetes mellitus and patients after cardiac transplantation. Ewing and colleagues1 tested both a fixed threshold of 50 ms and a variable threshold set at 6.25% of the previous NN interval. They recommended the 50 ms fixed threshold because it was “easier and simpler to measure than a percentage threshold”. However, results for fixed thresholds greater or less than 50 ms were not described. Subsequently, Bigger and colleagues2 introduced the pNN50 statistic, defined as NN50 count/total NN count—that is, the percentage of absolute differences in successive NN values > 50 ms. pNN50 has proved to be a useful HRV measure, providing diagnostic and prognostic information in a wide range of conditions.3–8
pNN50, however, is only one member of a larger family of HRV statistics, designated here as pNNx, where x > 0 ms. Surprisingly, in reviewing the literature, we were unable to find an answer to a basic question regarding pNN50 and other members of this family: how do other thresholds compare with the conventional 50 ms value in separating various groups under physiological and pathological conditions? We found that computing pNNx with x < 50 ms in both long and short term recordings consistently provides more robust discrimination between groups than the standard pNN50 measurement.
METHODS
Probability distributions for the family of pNNx statistics (where x ranges from 4–100 ms) were calculated from RR interval data obtained from 155 subjects. Primary comparisons between the following groups or conditions were made retrospectively: (a) healthy subjects versus those with congestive heart failure (CHF); (b) sleeping versus awake healthy subjects; and (c) young versus elderly healthy subjects. Only subjects with underlying normal sinus rhythm were analysed.
The data for the normal control group were obtained from 24 hour Holter monitor recordings of 72 healthy subjects (35 men and 37 women aged 20–76 years, mean 54.6) with ECG data sampled at 128 Hz. These recordings were from 18 subjects from the PhysioNet normal sinus rhythm database (www.physionet.org/physiobank/database/nsrdb), eight subjects from a Columbia-Presbyterian Medical Center database, and 46 subjects from a Washington University School of Medicine database.9–11 The data for the CHF group were obtained from 24 hour Holter recordings of 43 patients (28 men and 15 women aged 22–79 years, mean 55.5), 12 with New York Heart Association (NYHA) functional class I–II status, and 31 with class III–IV status. These data were from 14 subjects from the PhysioNet CHF database (www.physionet.org/physiobank/database/chfdb) with recordings sampled at 250 Hz and 29 subjects from a Columbia-Presbyterian Medical Center database with recordings sampled at 128 Hz.12,13
The sleeping and waking data sets were obtained from the 72 healthy subjects taking part in the CHF comparison. Sleeping hours were defined as the six continuous hours of lowest average heart rate; waking hours were defined as the six continuous hours of highest heart rate.
The healthy young and elderly groups consisted of 20 young healthy subjects (10 men and 10 women aged 21–34 years, mean 25.9) and 20 healthy elderly subjects (10 men and 10 women aged 68–85 years, mean 74.5). ECG data were recorded at 250 Hz for two hours in a resting state, as described previously (www.physionet.org/physiobank/database/fantasia).14
For each RR interval series, the absolute values of the differences in consecutive NN intervals were obtained. For each NN interval increment value the probability distribution of increments greater than that particular value was then calculated. Because of the different sampling frequencies of the data sets, each distribution was linearly resampled at 2 ms intervals to provide uniformly sampled points. Group means and standard deviations were then calculated at each resampled value of the distributions.
Further, the sampling rate of 128 Hz (7.8125 ms/sample) used in some of the Holter recordings leads to small round off errors in the determination of the NN intervals when reported to the standard 1 ms accuracy. Consequently, the NN intervals were quantised into 1 ms bins, causing stepwise jumps in the pNN distributions. To remove such artefactual discontinuities, each average distribution was smoothed using a five point moving window average. Probability (p) values for the differences between groups at each pNN value were then calculated using an unpaired Student's t test for the normal versus CHF and young versus elderly comparisons and a paired t test for the sleeping versus waking comparison. Significance was defined by p < 0.05.
RESULTS
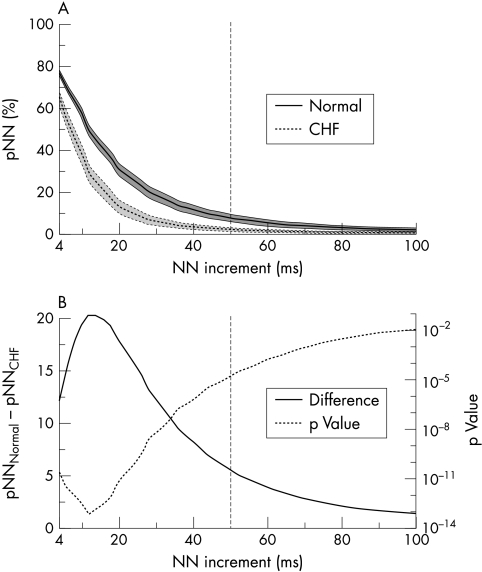
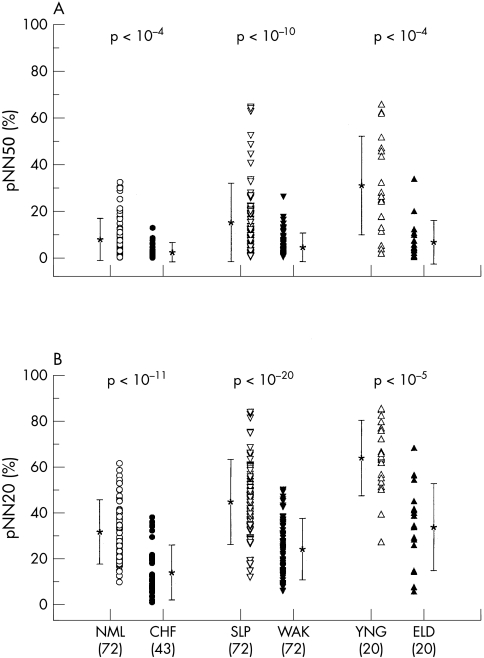
Consistent with previous reports, we observed significantly (p < 0.0001) higher pNN50 values for healthy subjects than for patients with CHF, for sleeping than for waking hours in healthy subjects, and for healthy young than for healthy elderly subjects (figs 1 and 2, table 1).1,7,10,11 Further, the mean and range of these pNN50 values were comparable with those previously reported. For all three comparisons, separation between the groups was improved at pNN values substantially less than 50 ms (fig 2): maximum separation was at pNN12 (49.8 (11.1)% v 29.6 (14.0)%, p < 10−13) for the normal versus CHF groups (fig 1) and at pNN12 (61.4 (13.7)% v 42.3 (11.9)%, p < 10−21) for the sleeping versus waking groups, respectively. For the healthy young versus the healthy elderly, maximum separation was at pNN28 (52.4 (18.2)% v 20.8 (15.0)%, p < 10−6). Comparable results were obtained for logarithmically transformed values of the pNN distributions.
Figure 1.
(A) Mean pNN distributions, showing the mean percentage of successive NN interval increments greater than each given increment (pNN) versus the NN increment. pNN was calculated for each subject in the normal and congestive heart failure (CHF) groups over a 24 hour period and the resulting distributions were resampled at 2 ms intervals and smoothed using a five point moving window average. The means and standard deviations were then calculated for each group at each resampled point. The solid line indicates the mean values for the group of healthy subjects (n = 72); the dotted line indicates the mean values for the group with moderate to severe CHF (n = 43). The shaded bands represent 2 SEM for these data sets. (B) Mean pNN distribution differences. The difference between the means of the normal group and the CHF group shown in (A) is plotted as a solid line and the p values for the separation between groups at different NN increments are plotted as a dotted line. The most significant separation between the two groups is around x = 12 ms, not at the traditional x = 50 ms (vertical dashed line).
Figure 2.
Comparison of (A) pNN50 with (B) pNN20 for subjects in the three primary comparison groups. Group means (*) and standard deviations are indicated alongside each data set. Probability (p) values computed using the Student's t test are indicated above each pair of comparison groups. The number of subjects in each group is given in parentheses below. More robust separation is obtained for pNN20 than pNN50 for each comparison. CHF, congestive heart failure; ELD, healthy elderly subjects; NML, healthy control subjects; SLP, healthy subjects during sleeping hours; WAK, healthy subjects during waking hours; YNG, healthy young subjects.
Table 1.
Comparison of pNN50 with pNN20
| Age (years) | |||||||
| Group | Number | Mean | Range | Duration (hours) | Heart rate (beats/min) | pNN50 (%) | pNN20 (%) |
| Normal | 72 | 54.6 | 20–76 | 24 | 76.7 (7.4) | 7.9 (7.8) | 31.3 (12.9) |
| CHF | 43 | 55.5 | 22–79 | 24 | 88.9 (12.9) | 2.3 (2.9) | 13.5 (10.8) |
| Sleeping | 72 | 54.6 | 20–76 | 6 | 63.2 (6.9) | 15.0 (15.6) | 44.2 (17.5) |
| Waking | 72 | 54.6 | 20–76 | 6 | 87.7 (9.6) | 4.2 (4.9) | 23.5 (12.2) |
| Young | 20 | 25.9 | 21–34 | 2 | 62.2 (8.5) | 30.7 (19.9) | 63.3 (15.4) |
| Elderly | 20 | 74.5 | 68–85 | 2 | 57.8 (8.7) | 6.3 (8.1) | 32.9 (17.8) |
Values are mean (SD).
Figure 2 shows p values for pNN50 and pNN20 statistics.
CHF, congestive heart failure.
Since the traditional pNN50 measure yielded significant separations between the above groups, we also compared subgroups of healthy elderly patients in the CHF comparison who were older than 65 years (n =22, age 66–76, mean 69.0) with younger CHF subjects who were younger than 55 years (n = 18, aged 22–54, mean 45.0). In this comparison, pNN50 failed to distinguish between these two groups (4.7 (5.3)% v 2.6 (3.9)%, p < 0.17), whereas pNN20 provided significant separation (25.1 (11.9)% v 13.9 (12.8)%, p < 0.007). We also compared data from the subgroup of the 12 CHF subjects with NYHA class I–II CHF with data from the 31 subjects with class III–IV CHF. pNN50 failed to distinguish these groups (3.4 (4.3)% v 1.9 (2.1)%, p < 0.13), whereas pNN10 yielded a significant separation (46.7 (14.4)% v 36.1 (14.4)%, p < 0.04).
DISCUSSION
The widely used and readily obtained pNN statistic is traditionally computed based on an empirical threshold of 50 ms.1–3 However, analysis of heart rate data obtained in different physiological and pathological states shows that separation between comparison groups is consistently improved by using threshold values substantially below 50 ms (figs 1 and 2). This observation indicates the importance of analysing relatively small variations in heart rate, with thresholds as low as 20 ms or less. Thresholds < 8 ms are likely to be of limited reliability for ECG data obtained with Holter monitors using standard sampling rates of 128 Hz, but may be useful at higher sampling rates.
These findings support the utility of evaluating the general pNN family of HRV statistics rather than selecting only the traditionally accepted pNN50 measure or other fixed thresholds. Subtle fluctuations in sinus rhythm heart rate increments quantified by pNNx < 50 ms appear to provide useful information about the very short term control of sinus rhythm dynamics in health and disease, presumably related to parasympathetic regulation.1–3
In summary, we examined a family of HRV statistics, termed pNNx, of which pNN50 is only one member. Enhanced discrimination between a variety of normal and pathological conditions is obtained by using values as low as 20 ms or less.
Acknowledgments
This work was supported by the National Institutes of Health, National Center for Research Resources (P41 RR13622); The G Harold and Leila Y Mathers Charitable Foundation; the Centers for Disease Control and Prevention (H75/CCH119124); The National Institute of Diabetes and Digestive and Kidney Diseases (DK 30583); and the National Institutes of Health, National Institute on Aging (AG08812). We thank Dr Luis Amaral for his insightful questions and Dr Phyllis Stein for her generous contribution of data and her helpful comments.
Abbreviations
CHF; congestive heart failure
HRV, heart rate variability
NYHA, New York Heart Association
REFERENCES
- 1.Ewing DJ, Neilson JMM, Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Br Heart J 1984;52:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigger JT Jr, Kleiger RE, Fleiss JL, et al. Components of heart rate variability measured during healing of acute myocardial infarction. Am J Cardiol 1988;61:208–15. [DOI] [PubMed] [Google Scholar]
- 3.Chakko S, Mulingtapang RF, Huikuri HV, et al. Alterations in heart rate variability and its circadian rhythm in hypertensive patients with left ventricular hypertrophy free of coronary artery disease. Am Heart J 1993;126:1364–72. [DOI] [PubMed] [Google Scholar]
- 4.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- 5.Tsuji H, Larson MG, Venditti FJ Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–5. [DOI] [PubMed] [Google Scholar]
- 6.Zuanetti G, Neilson JMM, Latini R, et al. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era–The GISSI-2 results. Circulation 1996;94:432–6. [DOI] [PubMed] [Google Scholar]
- 7.Szabo BM, van Veldhuisen DJ, van der Veer N, et al. Prognostic value of heart rate variability in chronic congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol 1997;79:978–80. [DOI] [PubMed] [Google Scholar]
- 8.Singh JP, Larson MG, Tsuji H, et al. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension. The Framingham heart study. Hypertension 1998;32:293–7. [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith RL, Bigger JT Jr, Steinman RC, et al. Comparison of 24-hour parasympathetic activity in endurance-trained and untrained young men. J Am Coll Cardiol 1992;20:552–8. [DOI] [PubMed] [Google Scholar]
- 10.Bigger JT, Fleiss LF, Steinman RC, et al. RR variability in healthy, middle-age persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 1995;91:1936–43. [DOI] [PubMed] [Google Scholar]
- 11.Stein PK, Ehsani AA, Domitrovich PP, et al. The effect of exercise training on heart rate variability in healthy older adults. Am Heart J 1999;138:567–76. [DOI] [PubMed] [Google Scholar]
- 12.Krum H, Bigger JT Jr, Goldsmith RL, et al. Effect of long-term digoxin therapy on autonomic function in patients with chronic heart failure. J Am Coll Cardiol 1995;25:289–94. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith RL, Bigger JT, Bloomfield DM, et al. Long-term carvedilol therapy increases parasympathetic nervous system activity in chronic congestive heart failure. Am J Cardiol 1997;80:1101–4. [DOI] [PubMed] [Google Scholar]
- 14.Iyengar N, Peng C-K, Morin R, et al. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol 1996;271:1078–84. [DOI] [PubMed] [Google Scholar]