Abstract
Objective: To examine the clinical outcome of percutaneous coronary intervention where the procedure was complicated by vessel perforation.
Setting: Tertiary referral centre.
Methods: The procedural records of 6245 patients undergoing coronary intervention were reviewed. In 52 patients (0.8%) the procedure was complicated by vessel perforation, ranging from wire exit to free flow of contrast into the pericardial space. The majority of lesions treated were complex (37% type B, 59% type C) and 9 of 52 (17%) were chronic occlusions. Ten patients (19%) received abciximab. Four underwent rotational atherectomy (8%).
Results: In 28 of 52 patients (54%) the perforation was benign and managed conservatively without the development of haemodynamically significant sequelae. In 24 of 52 (46%) a significant pericardial effusion ensued requiring drainage. Of these 24 procedures 6 had involved the treatment of a chronic occlusion (25%). Eight of the 24 patients were referred for emergency bypass surgery (33%), 3 of whom died. Of the remaining 16 not referred for surgery, 3 died. Of the 10 procedures complicated by vessel perforation where abciximab had been administered, 9 (90%) led to pericardial tamponade. Latterly 2 vessel perforations were successfully treated by the deployment of a covered stent.
Conclusions: Coronary artery perforation with sequelae during intervention is rare—26 of 6245 (0.4%). This complication was seen in the treatment of chronic occlusions, which are therefore not risk-free procedures. The development of pericardial tamponade carries a high mortality. While prompt surgical intervention may be life saving, expertise in the use of covered stents may provide a valuable rescue option for this serious complication. Caution should be exercised where coronary perforation occurs and abciximab has been used.
Keywords: vessel perforation, pericardial tamponade, coronary rupture
Vessel perforation during or following percutaneous coronary intervention (PCI) is a rare complication of this procedure. Previous reports cite an incidence of between 0.2–0.6%1–6 and while some of these series were reported during the era of balloon angioplasty alone, more recent studies have referred to intervention using newer devices including stents and rotational and directional atherectomy. Not all authors have included the full spectrum of pathology in their definition of vessel perforation. This may range from mere vessel puncture by the guidewire resulting in minimal dye staining without adverse haemodynamic consequences, to “wire exit”, to vessel rupture followed by brisk extravasation of blood and dye into the pericardial space leading to tamponade and abrupt haemodynamic collapse.
As the range of equipment available to the interventional cardiologist evolves, together with necessary operator expertise, more complex coronary anatomy is being treated percutaneously. Abrupt vessel closure in the catheterisation laboratory is often successfully resolved by stent deployment, together with parenterally administered platelet inhibitors. Therefore, the demand for emergent surgical rescue for this scenario has decreased.7 On the other hand, as calcified, tortuous, and occluded vessels are increasingly treated by percutaneous means, vessel perforation is likely to persist. Furthermore, widespread use of glycoprotein IIb/IIIa inhibitors introduces a compounding factor influencing the clinical consequences of perforation.
In this study we have analysed the prospectively recorded procedural data on all patients attending this institution for PCI between 1995 and 2001, and we report the incidence and outcome of vessel perforation in this cohort.
METHODS AND PATIENTS
A total of 6245 patients underwent PCI between 1995 and 2001. Their ages ranged from 20–95 years (median 64) and 4467 of them were men. The majority of lesions treated in these patients were classified as American College of Cardiology/American Heart Association class B or C. All procedural data were entered on to the catheterisation laboratory database. Procedures were performed according to conventional guidelines applicable at the time of treatment. The choice of equipment and access were determined by the operator. All patients received heparin at the time of intervention and all received aspirin afterwards unless a specific contraindication existed. Patients receiving an intracoronary stent were also treated with either ticlopidine or clopidogrel according to contemporary guidelines.
Coronary perforation was defined as evidence of extravasation of dye or blood from the coronary artery during or following the interventional procedure. This was determined either by angiographic appearances consistent with dye outside of the vessel lumen or by echocardiographic evidence of a pericardial effusion. Pericardial tamponade was defined by one or more of the following criteria: (a) systemic hypotension (systolic blood pressure < 90 mm Hg) with evidence of pulsus paradoxus on clinical or invasive assessment; (b) echocardiographic evidence of pericardial fluid collection, major respiratory variation in transmitral Doppler velocity, dilated inferior vena cava failing to collapse with inspiration, or right ventricular free wall collapse in diastole; and (c) angiographic evidence of significant collection of dye in the pericardial space. Where possible an emergency echocardiogram was performed when tamponade was suspected but, where the clinical scenario demanded it, pericardial drainage was carried out without prior imaging.
Statistical analysis
Where appropriate, groups were compared by the χ2 test and a value was considered significant if p < 0.05.
RESULTS
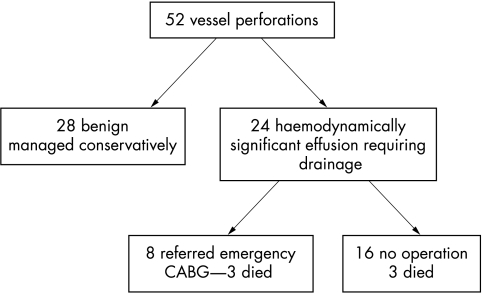
Over the six year period incorporating 6245 PCIs, 52 cases of vessel perforation were recorded (0.8%). Table 1 lists the baseline clinical and angiographic characteristics of the patients with surgery complicated by perforation. A relatively high proportion of the patients in this subset were women (48% v 28% in the study cohort). In 28 of the 52 patients (54%) the perforation was benign and conservative treatment alone was required. Outcome was good in all of these instances. In the remaining 24 of 52 interventions (46%) a haemodynamically significant pericardial effusion ensued requiring drainage. Figure 1 illustrates the subsequent management of this group of patients. Eight of the 24 (33%) were referred for emergency coronary artery bypass grafting, three of whom died. Patients were referred to surgery when pericardial drainage had not corrected the initial haemodynamic compromise or when persistent significant bleeding from the drainage catheter was observed. Of the remaining 16 patients who were not referred for surgery, three died (19%). Therefore, the total mortality in patients who developed pericardial tamponade was 6 of 24 (25%). Latterly in two cases the coronary perforations were treated by the deployment of a covered stent with good outcome in both instances. Six of the 24 cases (25%) had involved treatment of a chronic occlusion.
Table 1.
Baseline clinical and angiographic characteristics of patients with coronary perforation
| Age (years) | 52–82 (median 64) |
| Women | 25 (48%) |
| Diabetes | 4 (8%) |
| Hyperlipidaemia | 27 (52%) |
| Hypertension | 25 (48%) |
| Recent myocardial infarction (<1 week) | 5 (10%) |
| Previous CABG | 3 (6%) |
| Lesion morphology (ACC/AHA) | |
| Type A | 2 (4%) |
| Type B | 19 (37%) |
| Type C | 31 (59%) |
| Chronic occlusions | 9 (17%) |
| Impaired left ventricular function (<30%) | 4 (8%) |
ACC/AHA, American College of Cardiology/American Heart Association; CABG, coronary artery bypass graft.
Figure 1.
Clinical management of patients with coronary vessel perforation following percutaneous intervention.
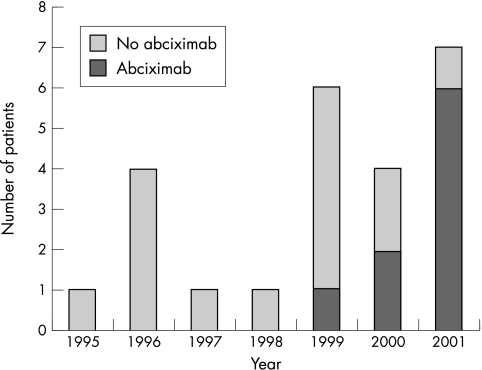
Table 2 lists the device used for intervention, the type of guidewire used during the procedures that resulted in perforation, and the dose of heparin administered during the procedure. Only four instances of coronary perforation were reported during or after rotational atherectomy, three of which resulted in tamponade. This was only 1.2% (3/254) of the total number of rotational atherectomy procedures performed during this period. This did not differ significantly from the incidence of tamponade in cases where atherectomy was not used (3/254 v 21/5991 (0.4%), NS). Figure 2 shows the number perforations resulting in significant pericardial effusion per year and the proportion of these procedures during which the glycoprotein IIb/IIIa antagonist abciximab was used. Of the 10 cases of perforation where abciximab was used, 9 (90%) were complicated by a significant pericardial effusion and only one was benign.
Table 2.
Drugs and equipment used where intervention was complicated by coronary perforation
| Device used | |
| Balloon only | 22 (42%) |
| Intracoronary stent | 26 (50%) |
| Thrombectomy device | 0 |
| Rotational atherectomy | 4 (8%) |
| Type of guidewire | |
| Floppy tip (non-hydrophilic) | 22 (42%) |
| Intermediate (non-hydrophilic) | 14 (27%) |
| Standard (non-hydrophilic) | 2 (4%) |
| Hydrophilic | 14 (27%) |
| Heparin dose (U) | |
| 5000 | 13 (25%) |
| 10 000 | 36 (69%) |
| >10 000 | 3 (6%) |
| Glycoprotein IIb/IIIa antagonist | 10 (19%) |
Figure 2.
Number of patients where coronary vessel perforation resulted in haemodynamically significant pericardial effusion per year.
In five instances pericardial tamponade became clinically manifest > 2 hours following the procedure. In four of these five cases (80%) abciximab had been administered. In three of the five cases a hydrophilic wire had been used, in one an intermediate tip wire, and in one a floppy tipped wire. Vessel perforation was not clinically or angiographically obvious at the conclusion of the procedure in any of these five cases and the mechanism of vessel perforation was believed to be distal branch puncture with the guidewire tip. In two separate instances tamponade became manifest in the catheterisation laboratory recovery area between 30–60 minutes after the procedure. Abciximab had been used in both of these cases and platelet transfusion was carried out urgently. In the remaining 17 cases of pericardial tamponade clinical presentation occurred at the time of the procedure.
DISCUSSION
In this study we have reported an overall incidence of coronary perforation of 0.77% during or following PCI. The incidence of perforation that resulted in a haemodynamically significant pericardial effusion is less at 0.38%. This is consistent with previous reports,1–6 although in these studies the definition of vessel perforation varied. Gruberg and colleagues reported an incidence of 0.29% but in their study the clinical spectrum of the cases was not clearly defined.1 Approximately a third appear to have developed cardiac tamponade. Von Sohsten and colleagues on the other hand recorded 15 cases of cardiac tamponade occurring in 6999 coronary interventions (0.21%) but benign perforations that were treated by conservative means were not described.2 Their case mix included instances where tamponade had arisen from damage to the right ventricle by a temporary pacing wire rather than the coronary artery per se. Some of these investigations were conducted over a decade ago in the era of balloon angioplasty alone and clearly the equipment used for intervention has evolved since that time. This is discussed further below.
Atherectomy
Von Sohsten and colleagues reported on pericardial tamponade during the “new device era”.2 They reported eight cases of tamponade in 743 rotational atherectomy procedures (1.1%). Gruberg and colleagues, analysing a subset of their patients treated between 1995 and 1999, reported an incidence of 0.4%.1 Reisman and colleagues described the outcome of 2953 atheroablative procedures between 1988 and 1994 incorporating two chronological registries.8 In the first registry 23 of 2953 cases were complicated by perforation (0.8%) and in the second the incidence was one in 200 (0.5%). Our findings were similar to these previous reports in that three of 243 rotational atherectomy cases were complicated by tamponade (1.2%) and a further single case of perforation was managed conservatively. Perforation is a rare complication of rotablation even though patients selected for this procedure often have calcified coronary arteries (one of the main indications for the technique). Calcification is a well recognised risk factor for perforation during PCI, irrespective of the equipment used.9,10
Platelet inhibitors
The use of glycoprotein IIb/IIIa inhibitors (in particular abciximab) during PCI has increased significantly over the past two to three years. This is particularly relevant to centres in the UK following publication of the National Institute for Clinical Excellence recommendations.11 The rationale for this increase is evidence for improved short and intermediate term outcome after PCI, particularly in diabetic patients and in those where an occluded vessel is treated.12–14 Abciximab is a potent platelet inhibitor and these effects are manifest from the time of bolus administration, persist throughout the course of infusion, and may last for 12 hours after discontinuation.15,16 Therefore, the severity of any bleeding complication arising from the intervention is likely to be accentuated by this agent.
As indicated in fig 2 an appreciable proportion of the cases of tamponade in our cohort reported in 2000 and 2001 followed procedures where abciximab had been used. Furthermore, 9 of 10 (90%) perforations that were associated with this agent required pericardial drainage and 4 of these patients developed clinical signs of tamponade after a delay of > 2 hours after the procedure. Retrospective review of the angiograms of these late presentations found minimal or no evidence of dye extravasation from the coronary vessel. The mechanism of the leak into the pericardial space was deemed to be a distal vessel puncture by the guidewire and the degree of bleeding was augmented by evolving platelet inhibition. Von Sohsten and colleagues reported only three cases of perforation and tamponade associated with this agent but indicated that it was used in only 20% of PCI procedures in their institution.2 This compares with a current usage of approximately 60% of all cases in our unit, perhaps accounting for the higher incidence of tamponade in our study.
These results serve as a cautionary note about the use of abciximab. This applies in the treatment of chronic occlusions where the potential for wire exit is likely to be higher than in a conventional case. One approach is to withhold the administration of abciximab until the occlusion has been safely crossed with the guidewire and the operator is confident that the tip of the wire is seated intraluminally in the distal bed of the vessel. Our findings also suggest that in all cases caution should be exercised in positioning the tip of the guidewire distally, particularly if a hydrophilic wire is used. Where there is evidence of a perforation during a procedure where abciximab has been administered, careful clinical observation is prudent. Most importantly, a platelet transfusion should be strongly considered.
Covered stents
There are now several reports of the use of covered stents to seal coronary perforations with good effect.17–19 Clearly this is more effective if the perforation is located in the proximal or mid-portion of the index vessel and the guidewire is correctly positioned distally in the true lumen. In two cases in our series these devices were successfully deployed and dramatic extravasation of dye and blood was terminated, with good clinical outcome and without the need for surgical intervention. One limitation of the covered stent is a lack of flexibility. Thus, rapid deployment in calcified and tortuous vessels may prove technically difficult, particularly in emergency conditions. Nevertheless, they provide a valuable rescue option in selected instances and should be considered early in any treatment algorithm.
Study limitations
Although the data reported in this study were recorded prospectively, this report is a retrospective review of our experience over a six year period. As such, treatment options have not been compared in this study and little statistical analysis has been required.
Conclusions
Coronary artery perforation with sequelae during intervention is rare. This complication is seen in the treatment of chronic occlusions, which should not be regraded as risk-free procedures. The use of newer devices such as rotational atherectomy to treat difficult coronary anatomy carries a low risk of coronary perforation. The development of pericardial tamponade carries a high mortality. While prompt surgical intervention may be life saving, expertise in the use of covered stents may provide a valuable rescue option for this serious complication. Caution should be exercised where coronary perforation occurs and abciximab has been used.
REFERENCES
- 1.Gruberg L, Pinnow E, Flood R, et al. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol 2000;86:680–2. [DOI] [PubMed] [Google Scholar]
- 2.Von Sohsten R, Kopistansky C, Cohen M, et al. Cardiac tamponade in the new device era: evaluation of 6999 consecutive percutaneous coronary interventions. Am Heart J 2000;140:279–83. [DOI] [PubMed] [Google Scholar]
- 3.Kimbris D, Iskandrian AS, Goel I, et al. Transluminal coronary angioplasty complicated by coronary artery perforation. Cathet Cardiovasc Diagn 1982;8:481–7. [DOI] [PubMed] [Google Scholar]
- 4.Grollier G, Bories H, Commeau P, et al. Coronary artery perforation during coronary angioplasty. Clin Cardiol 1986;9:27–9. [DOI] [PubMed] [Google Scholar]
- 5.Topaz O, Cowley MJ, Vetrovec GW. Coronary artery perforation during angioplasty; angiographic detection and demonstration of complete healing. Cathet Cardiovasc Diagn 1992;27:284–8. [DOI] [PubMed] [Google Scholar]
- 6.Nassar H, Hasin Y, Gotsman MS. Cardiac tamponade following coronary arterial rupture during coronary angioplasty. Cathet Cardiovasc Diagn 1991;23:177–9. [DOI] [PubMed] [Google Scholar]
- 7.Reinecke H, Fetsch T, Roeder N, et al. Emergency coronary artery bypass grafting after failed coronary angioplasty: what has changed in a decade? Ann Thorac Surg 2000;70:1997–2003. [DOI] [PubMed] [Google Scholar]
- 8.Reisman M, Harms V, Whitlow P, et al. Comparison of early and recent results with rotational atherectomy. J Am Coll Cardiol 1997;29:353–7. [DOI] [PubMed] [Google Scholar]
- 9.Reimers B, Von Birgelen C, van der Giessen WJ, et al. A word of caution on optimizing stent deployment in calcified lesions: acute coronary rupture with tamponade. Am Heart J 1996;131:192–4. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SL, Colombo A, Nakamura S, et al. Benefit of intracoronary ultrasound in the deployment of Palmaz-Schatz stents. J Am Coll Cardiol 1994;24:996–1003. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Clinical Excellence. Guidance on the use of glycoprotein IIb-IIIa inhibitors in the treatment of acute coroanry syndromes. NICE technology appraisal guidance, no 12. London: NICE, 2000.
- 12.Topol EM, Lincoff AM, Califf RM, et al. Randomised placebo-controlled and balloon angioplasty controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet 1998;352:87–92. [DOI] [PubMed] [Google Scholar]
- 13.Lincoff AM. Sustained supression of ischemic complications of coronary intervention by platelet GPIIb/IIIa blockade with abciximab: one year outcome in the EPILOG trial. Circulation 1999;99:1951–8. [DOI] [PubMed] [Google Scholar]
- 14.Topol EJ, Califf RM, Weisman HF, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC investigators. Lancet 1994;343:881–6. [DOI] [PubMed] [Google Scholar]
- 15.Tcheng JE, Ellis SG, George BS, et al. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high-risk coronary angioplasty. Circulation 1994;90:1757–64. [DOI] [PubMed] [Google Scholar]
- 16.Kleiman NS. Pharmacology of the intravenous platelet receptor glycoprotein IIb-IIIa antagonists. Coron Artery Dis 1998;9:603–16. [DOI] [PubMed] [Google Scholar]
- 17.Campbell PG, Hall JA, Harcombe AA, et al. The Jomed covered stent graft for coronary artery aneurysms and acute perforation: a successful device which needs careful deployment and may not reduce restenosis. J Invasive Cardiol 2000;12:272–6. [PubMed] [Google Scholar]
- 18.Casella G, Werner F, Klauss VV, et al. Successful treatment of coronary artery perforation during angioplasty using a new membrane-coated stent. J Invasive Cardiol 1999;11:622–6. [PubMed] [Google Scholar]
- 19.Nageh T, Thomas MR. Coronary-artery rupture treated with a polytetrafluoroethylene-coated stent. N Engl J Med 2000;342:1922–4. [DOI] [PubMed] [Google Scholar]