Abstract
The biological basis for developmental dyslexia remains unknown. Research has suggested that a fundamental deficit in dyslexia is the inability to process sensory input that enters the nervous system rapidly and that deficits in processing rapid acoustic information are associated with impaired reading. Functional magnetic resonance imaging (fMRI) was used to identify the brain basis of rapid acoustic processing in normal readers and to discover the status of that response in dyslexic readers. Normal readers showed left prefrontal activity in response to rapidly changing, relative to slowly changing, nonlinguistic acoustic stimuli. Dyslexic readers showed no differential left frontal response. Two dyslexic readers participated in a remediation program and showed increased activity in left prefrontal cortex after training. These fMRI results identify left prefrontal regions as normally being sensitive to rapid relative to slow acoustic stimulation, insensitive to the difference between such stimuli in dyslexic readers, and plastic enough in adulthood to develop such differential sensitivity after intensive training.
Developmental dyslexia is a reading disorder that affects between 5% and 17% of the population (1). It is characterized by reading difficulty in those who otherwise have the intelligence, motivation, and schooling necessary for accurate and fluent reading (1, 2). Neurobiological and genetic influences on dyslexia have been found from multiple research methods, including postmortem anatomy (3), neuroimaging [positron emission tomography (PET) (4–8) and functional MRI (fMRI) (9–11)], electro- and magneto-encephalography (12–14), and genetic linkage studies (15). These studies are representative of many that suggest a biological basis for dyslexia; however, its fundamental nature remains unknown and an active topic of research.
Developmental dyslexia seems on its face to be a primarily visual problem, and early explanations characterized dyslexia as a visual disorder typified by reversal of letters and words. These theories, however, have not been supported by the evidence (1). Although dyslexic individuals do exhibit some abnormalities of visual processing (particularly for rapidly transient visual stimuli) (9–10, 16–18), a strong consensus has developed that the central difficulty in dyslexia is related to the processing of speech sounds, known as phonological processing (1, 2). Sensitivity to speech sounds (phonemes) normally develops in infancy and continues to be shaped by experience, especially in the first year of life (19). Dyslexic individuals are particularly impaired at an aspect of phonological processing known as phonemic awareness, which is the ability to consciously decompose words into their constituent speech sounds (1, 2). Phonemic awareness develops as a child learns to read: The emerging reader (especially of alphabetic languages such as English) requires phonemic awareness to map orthographic representations onto existing spoken language representations (20, 21). Dyslexia appears to involve a problem in learning to relate visual input to phonological representations.
Some researchers have suggested that the phonological processing deficit in dyslexia may reflect a more fundamental deficit in the processing and integration of rapidly successive and transient signals in the nervous system (the rapid processing hypothesis) (22–24). According to the rapid processing hypothesis, deficits in processing transient rapid acoustic signals impair the ability to discriminate acoustic cues that are necessary to distinguish phonemes. This impairment compromises the development of robust and stable phonological representations, which in turn leads to the difficulties in phonological processing observed in dyslexia (22). This hypothesis has been supported by behavioral research (for reviews, see refs. 25 and 26) reporting that dyslexic readers have deficits in processing rapidly transient acoustic and visual stimuli (9–10, 16–18, 22, 27, 28) and that the deficits are correlated across modalities (29). There is, however, debate about the precise relations between nonspeech acoustic deficits and reading impairments (30, 31).
Neuroimaging of reading or reading related tasks have demonstrated reduced activity in the left perisylvian region in dyslexia (4, 6–8, 11). Additionally, studies have suggested a functional (32) and structural (33) disconnection between frontal and temporal language regions in adult dyslexics. To date, however, no study using fMRI or PET has examined the neural response to rapidly changing nonlinguistic auditory stimuli in dyslexic readers to test whether, as the rapid processing hypothesis predicts, this group exhibits differences in the neural response to rapidly changing acoustic information.
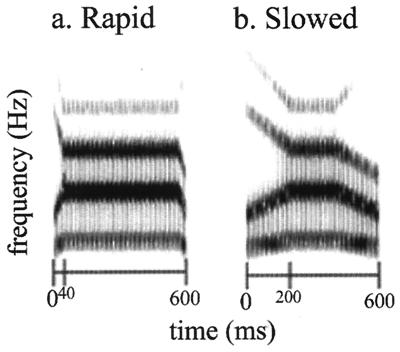
The goal of this study was to discover whether in dyslexia there is a disruption in the neural response to rapid nonlinguistic acoustic stimuli. Computer-synthesized complex acoustic stimuli were presented that had rapid or slow acoustic transitions at the front and tail of a central steady-state period. The stimuli were nonspeech analogues specifically designed to mimic the spectro-temporal acoustic changes that characterize consonant–vowel–consonant syllables but did not correspond to any actual or possible speech stimulus (as in ref. 34) (Fig. 1). Subjects performed a pitch discrimination task while stimuli alternated between blocks of rapid and slow nonspeech analogues. Subjects were instructed to press a button for high-pitched, but not for low-pitched, stimuli. The pitch discrimination task was unrelated to the manipulation of temporal acoustic changes, so any difference in neural response would reflect an incidental or automatic sensitivity to rapid acoustic changes. Whole-brain fMRI was performed while subjects (Table 1) made these pitch judgments for rapid and slow nonverbal sounds.
Figure 1.
Nonspeech analogues (34). Spectrograms of stimuli with frequency (Hz) on the vertical axis and time (ms) on the horizontal. (a) Rapid stimuli are shown. The spectro-temporal structure of the rapid stimuli was similar to that of consonant–vowel–consonant speech syllables, with very rapid acoustic changes occurring over 40 ms surrounding a 520-ms steady-state period. (b) Slow stimuli are shown. The duration of the acoustic transition was extended to 200 ms.
Table 1.
Subject characteristics
| Variable | Normal readers (n = 10) | Dyslexic readers (n = 8) | P value |
|---|---|---|---|
| Age, years | 32 (11) | 28 (13) | NS |
| Education, years | 14 (3) | 13 (2) | NS |
| Handedness | |||
| % right-handed | 90 | 75 | NS |
| Annett laterality score | 14.7 | 10.5 | NS |
| Gender, % male | 90 | 88 | NS |
| Nonverbal IQ* | 100 (15) | 90 (7) | 0.10 |
| Woodcock Reading Mastery* | |||
| Word ID (real word reading) | 107 (10) | 83 (12) | <0.001 |
| Word attack (nonword reading) | 108 (10) | 89 (16) | <0.01 |
| Rapid Auditory Processing | |||
| Threshold ISI (ms) for three-tone ordering | 38 | 267 | <0.001 |
Data are means (standard deviation). NS, not significant.
Standard score is 100 (15).
Materials and Methods
Subjects.
Eight adults with a history of developmental dyslexia and 10 matched controls with no history of reading or language disorders were selected. Standardized reading tests were given to confirm the case history classification and group difference on reading measures (Table 1). All were physically healthy and free of any history of neurologic disease. One dyslexic subject was receiving medication for bipolar disorder. (Exclusion did not alter results, and so results include this subject.) Informed consent was obtained after the nature and possible risks of the experiment were explained.
Task and Stimuli.
Subjects were instructed to press a button for high-pitched (250 Hz F0) but not for low-pitched stimuli (125 Hz F0). Sounds lasting 600 ms were presented every 1,050 ms and alternated [20 total blocks (16.8 s) of 16 items] between counterbalanced blocks of either rapid (40 ms) or slow (200 ms) stimuli (320 items and a total scan length of 336 s). In each block, half were high pitched with a pseudo-random ordering.
fMRI Acquisition.
Images were acquired with a 1.5-T GE Signa whole-body scanner with a whole-head elliptical coil by means of a single-shot gradient-echo T2* spiral, with one interleave (35) [TR = 2,100 ms; TE = 40 ms; flip angle = 85°; FOV = 22 cm, 20 sagittal slices (6-mm slice thickness and 1-mm skip)]. Three-dimensional SPGR T1 volume (TE = minimal full; flip angle = 15°; FOV = 24 cm) and T1 spin-echo structural images (colocalized to the fMRI slices) were collected as a substrate to overlay functional data. Four dummy images (8.4 s) were collected before stimulus presentation to allow for dissipation of gradient-induced auditory cortical activation (36). A bite-bar was used, and the motion artifact was corrected with AIR 3.0 (37). Responses were collected during scanning with a hand-held fiber-optic response button. Auditory stimuli were delivered binaurally by using a Resonance Technology (Van Nuys, CA) system.
fMRI Analysis.
Data were analyzed by use of SPM96 (Wellcome Department Cognitive Neurology, London, U.K.) implemented in MATLAB (Mathworks, Natick, MA). Images were normalized into a standard space (MNI standard brain) by aligning each image to a normalized template using a nine-parameter linear transformation and then smoothed by using a 10-mm full-width at half-maximum Gaussian kernel. Data were analyzed according to a mixed-effects general linear model, treating subjects as a random effect to allow population inference (38). For each subject, adjusted mean images were created for each condition after removing global signal and low-frequency covariates. Condition effects were estimated according to the general linear model at each voxel. Contrasts were used to compare the effect of stimulus type (rapid vs. slow) along with the stimulus type by group interaction. Voxels were considered statistically significant at an uncorrected P < 0.005.
Training and Testing.
Training.
fast forword (Scientific Learning, Berkeley, CA) training consisted of seven adaptive computer exercises designed to improve rapid successive processing by using linguistic and nonlinguistic stimuli, as well as syntactic and semantic skills, for 100 min a day, 5 days a week, for an average of 33 training days (23, 24, 39).
Testing.
Rapid auditory processing used a three-tone identification task with 20-ms tones and a decreasing interstimulus interval (ISI) to determine 75% threshold ISI needed for detection. Auditory language comprehension used SCAN-A (The Psychological Corporation, Harcourt Brace). Reading comprehension used the Reading Quotient subtest of Gray's Oral Reading Test. Word reading used the Word ID subset of Woodcock Reading Mastery Tests [Revised (1987), American Guidance Service, Circle Pines, MN]. Handedness was tested as described in ref. 40. Nonverbal IQ was measured with the Matrix Analogies Test [expanded form (1985), J.A. Naglieri, Harcourt Brace].
Results
Behavioral Results.
For pitch discrimination performance, normal readers were slightly more accurate overall than were dyslexic readers [percent correct normal readers: 95% ± 1% (mean ± SEM) for rapid and 98% ± 2% for slow nonspeech analogues; percent correct dyslexic readers: 92% ± 3% for rapid and 93% ± 5% for slow nonspeech analogues, P < 0.01]. A small reduction in pitch discrimination in dyslexia has been reported (41). Accuracy was slightly but reliably greater for slow than for rapid stimuli (P < 0.01). Importantly, normal readers were not disproportionately more accurate for the rapid stimuli; there was no interaction between speed of the transition (rapid vs. slow) and group (normal vs. dyslexic reader) [F(1,16) = 2.95, P > 0.1]. Responses for correct trials were significantly faster for rapid than slow stimuli (P < 0.01) [reaction time for normal readers: 510 ± 70 ms (mean ± SEM) for rapid and 537 ± 64 ms for slow stimuli; reaction time for dyslexic readers: 468 ± 39 ms for rapid and 498 ± 48 ms for slow stimuli]. Thus, performance with the rapid stimuli was inferior by an accuracy measure but superior by a latency measure (speed–accuracy tradeoff). Response times did not differ between dyslexic and normal readers [F(1,16) = 2.38, P > 0.1], and there was no interaction between group and stimulus type [F(1,16) = 0.04, P > 0.1]. The similarity in reaction times between the two groups and the lack of interactions between group and stimulus type in both accuracy and reaction time suggest that any brain activation differences were not secondary to issues of differential task difficulty between the two groups.
fMRI Results.
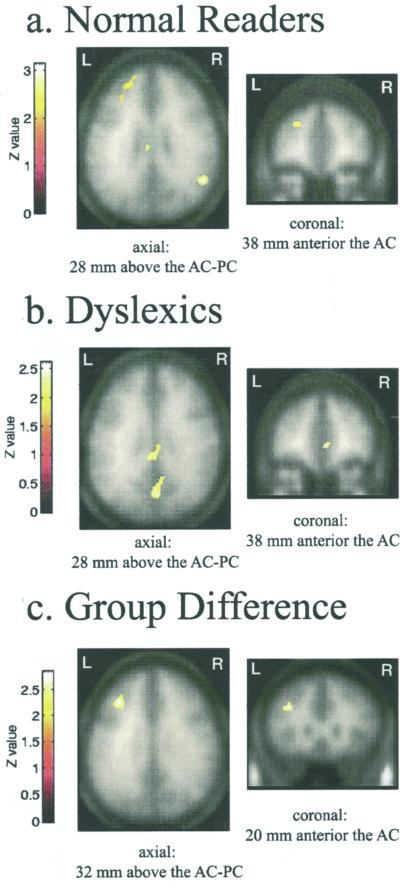
We obtained evidence for a specific disruption of the neural response to rapidly transient acoustic stimuli in adults with developmental dyslexia. Statistical analysis of the fMRI data revealed five brain regions in the normal readers that were more active during the blocks of rapid, than slow, nonspeech analogues (Table 2). The largest activation was in the left prefrontal region, between the middle and superior frontal gyri in Brodmann area 46/10/9 (Fig. 2a). Analysis of the dyslexic readers revealed no left frontal response to the rapid, relative to the slow, stimuli (Fig. 2b and Table 2). Individual analyses revealed that 9 of 10 normal readers exhibited a left frontal response to the rapid stimuli, whereas only 2 of the 8 eight dyslexic readers had any left frontal activity for this comparison. Analysis for trends of activity, by using a liberal threshold of P = 0.1, showed no differential activity for rapid relative to the slow stimuli in the dyslexic group in the left frontal cortex.
Table 2.
Stereotactic locations of significant activations for rapid nonspeech analogues compared to slow nonspeech analogues for each group and the differences between groups
| Group/region | Voxels, no. | BA | X | Y | Z | P value | Max Z |
|---|---|---|---|---|---|---|---|
| Normal readers | |||||||
| L middle/superior frontal gyri | 247 | 46/10/9 | −28 | 38 | 28 | 0.004 | 2.62 |
| R middle temporal gyrus | 146 | 39 | 50 | −62 | 28 | 0.001 | 3.19 |
| Bi cingulate gyrus | 116 | 23 | −6 | −26 | 28 | 0.004 | 2.78 |
| R medial frontal gyrus | 85 | 6 | 12 | 2 | 60 | 0.001 | 3.26 |
| R middle/superior temporal gyri | 65 | 21/38 | 40 | −8 | −12 | 0.003 | 2.78 |
| Dyslexic readers | |||||||
| Bi cingulate gyrus | 1519 | 31 | −4 | −34 | 36 | 0.001 | 2.83 |
| Bi anterior cingulate gyrus | 408 | 24 | 2 | 38 | 0 | 0.003 | 2.76 |
| R middle temporal gyrus | 333 | 21 | 58 | −48 | 8 | 0.002 | 2.96 |
| R cerebellum | 239 | N/A | 22 | −60 | −28 | 0.004 | 2.68 |
| L cerebellum | 229 | N/A | −16 | −40 | −28 | 0.000 | 3.38 |
| L middle temporal gyrus/ | 81 | 21 | −50 | −2 | −16 | 0.000 | 3.63 |
| anterior temporal pole | 21/38 | −56 | 0 | −32 | 3.30 | ||
| medial cerebellum | 55 | N/A | −2 | −72 | −44 | 0.000 | 3.67 |
| Group difference | |||||||
| L middle/superior frontal gyri | 129 | 46/10/9 | −36 | 20 | 32 | 0.002 | 2.87 |
| R cerebellum (posterior lobe) | 63 | N/A | 40 | −78 | −40 | 0.002 | 2.95 |
XYZ coordinates refer to the number of millimeters from AC (anterior commissive) by using MNI standard brain coordinates. BA, putative Brodmann area; L, left; R, right; Bi, bilateral; N/A, not applicable.
Figure 2.
fMRI response to rapid auditory stimuli in normal and dyslexic readers (P < 0.025). (a) Normal readers (n = 10) show significant difference between rapid and slow nonspeech analogues in left prefrontal cortex. Normalized, averaged functional maps are overlaid on the smoothed averaged anatomies of 10 subjects. (b) Dyslexic readers (n = 8) show (on the smoothed averaged anatomies of dyslexic readers) no differential left frontal response to rapid stimuli. (c) Group difference in brain activity. Region shows significant group by stimulus interaction; normal readers had more activity than the dyslexic readers for the rapid versus slow comparison. AC-PC, anterior-posterior commissive plane.
Neural responses to rapidly changing stimuli in the dyslexic and control groups were compared directly. This analysis confirmed a disruption in the left frontal response in the dyslexic readers. The primary group difference in brain activity elicited by the rapid nonspeech analogues (such that normal readers had greater activity than dyslexic readers) was in the left prefrontal cortex, astride the middle and superior frontal gyri in Brodmann areas 46/10/9 (Fig. 2c and Table 2).
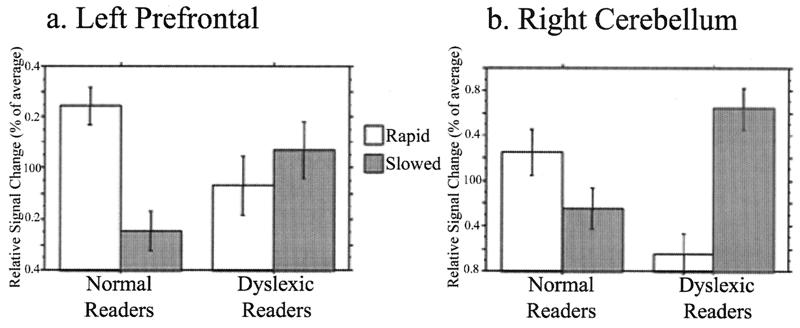
A functionally defined region of interest (ROI) in the left prefrontal cortex [created from the cluster of voxels that were significantly more active (P = 0.05) during the rapid than the slow blocks in the normal readers] was used to interrogate both groups' neural response to rapid auditory stimuli. This analysis showed an interaction between group and stimulus [F(1,16) = 5.78, P < 0.05]. Normal readers had significantly increased activity in this region during the rapid, as compared with slow, stimuli, whereas dyslexic readers had no difference in activity between stimulus types (Fig. 3a).
Figure 3.
Region of interest analysis of group differences in fMRI response to rapid auditory stimuli. Relative signal (percent of average significantly activated pixels) on vertical axes. Relative responses to blocks of rapid and slow stimuli are shown by open and shaded bars, respectively (error bars, SEM). Repeated measures analysis of variance (ANOVA) used to determine interactions between group (normal vs. dyslexic readers) and stimulus (rapid vs. slow). Two-tailed t tests were performed post hoc. (a) Left prefrontal cortex data are shown. Normal readers had significantly increased activity for rapid compared with slow nonspeech analogues (P < 0.001), whereas dyslexic readers had no significant difference in activity for the two stimulus types (P > 0.1). (b) Right cerebellar hemisphere data are shown. Dyslexic readers had more activity for slow than for rapid nonspeech analogues (P < 0.001), whereas normal readers showed the opposite trend (P = 0.06).
The group comparison also revealed the right posterior cerebellum as a region where dyslexic and normal readers differed in their neural response to the rapid nonspeech analogues (Table 2). To determine the nature of the interaction, a region of interest analysis was performed, by using the region defined by the group comparison as a functional ROI to interrogate both groups. The analysis showed a significant interaction between group and stimulus [F(1,16) = 2.4, P < 0.0001]). Dyslexic readers had significantly more activity for the slow than the rapid stimuli, whereas normal readers had an opposite pattern of activation with greater activity for rapid than slow stimuli (P = 0.06; Fig. 3b). The marginal statistical significance in the normal readers explains why this activation was not identified in the original results.
To consider the possible influence on activation of the small difference in pitch discrimination between groups, we performed a secondary analysis with groups matched for performance [normal (n = 5) and dyslexic (n = 4) readers had same mean accuracy (95.5% and 95.6% correct, respectively) and same range (94.7–96.3% correct)]. These performance-matched groups still showed the same group difference in response to rapid stimuli in both the left frontal and right cerebellar ROIs. In the left frontal region, normal readers had greater activity for rapid than slow stimuli (P < 0.05), and dyslexic readers had no difference between rapid and slow stimuli (P > 0.1). Even with the reduced power of only nine subjects, the interaction between group and stimulus type showed the same trend as in the larger group [F(1,7) = 4.28, P = 0.07]. In right cerebellum, normal readers had greater activity for rapid than slow stimuli (P = 0.05), and dyslexic readers had greater activity for slow than rapid stimuli (P < 0.01), with a significant interaction between stimulus and group [F(1,7)=60.3, P < 0.001]. Thus, the group difference in neural response to rapid stimuli was not a function of any group difference in pitch-discrimination accuracy.
A test of rapid auditory processing was performed outside the scanner by six dyslexic and eight normal readers (four subjects' data were not recorded accurately) in which a 75% threshold ISI in ordering three tones was determined for each subject. Dyslexic readers had a higher threshold ISI (267 ms vs. 38 ms; Table 1). Differential brain activity in the left frontal ROI was correlated with rapid auditory processing ability (the lower the threshold ISI, the greater the difference between activity for the rapid than the slow stimuli; R2 = 0.5, P = 0.007). Differential brain activity was not correlated with rapid processing in the right cerebellar ROI (R2 = 0.2, P > 0.1).
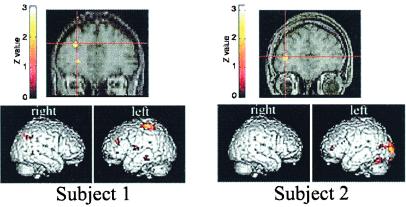
A question of practical and theoretical interest was whether the abnormal response seen in the dyslexic readers was fixed (due to either genetic and/or experiential factors) or plastic and, therefore, amenable to training in adulthood. In the present study, three of the eight dyslexic subjects underwent a training program (23, 24, 39) designed to improve rapid auditory processing and returned for fMRI retesting. Training did not include exposure to the nonspeech analogues or task used in the fMRI experiment. These three subjects were the only ones who both completed the training program as designed and agreed to undergo a follow-up fMRI session. When these subjects' brain responses to rapid nonspeech analogues were compared before and after training, two (Subjects 1 and 2) showed significantly increased activity in left prefrontal cortex (Fig. 4). These two individuals also showed significant improvement on both rapid auditory processing and auditory language comprehension tests after training, whereas the individual who did not show a brain response to rapid stimuli after training failed to show these behavioral improvements (Fig. 4). None of the subjects showed an effect of training in the right cerebellum.
Figure 4.
Training effects in brain responses to rapid auditory stimuli. Subjects 1 (Left) and 2 (Right) showed a training effect in the left frontal cortex, with greater activation for rapid than for slow stimuli after training. Regions that show a significant interaction of training (before and after) and stimulus (rapid and slow) are shown (P < 0.025) overlaid on the individuals' normalized anatomy. Spatial extent and location of all activity are shown below for both subjects. Behavioral improvement: Rapid auditory processing improvement for Subjects 1 and 2 were 82 and 164 ms, respectively; Subject 3's (not shown) improvement was only 31 ms. Auditory language comprehension improvement for Subjects 1 and 2 were 25 and 29 points respectively; Subject 3's improvement was only 8 points. All subjects improved on written language comprehension (gains of 15, 21, and 18 points, respectively) and more modestly on word reading (gains of 5, 7, and 6 points, respectively).
Discussion
This brain imaging study shows both a disrupted neural response to rapid auditory stimuli and its location in dyslexic adults. The study provides evidence for three main findings. (i) The left prefrontal cortex (specifically, Brodmann areas 46/10/9 along the middle and superior frontal gyri) is sensitive to rapidly changing, compared with slowly changing, nonverbal acoustic stimuli. The correlation between superior rapid auditory processing behaviorally and greater activation in left prefrontal cortex provides additional support for this area being specifically involved in processing rapid acoustic information. (ii) This study shows this sensitivity to rapid relative to slow nonverbal auditory stimuli in the left prefrontal cortex is disrupted and essentially absent in adult dyslexics. This finding suggests that rapid auditory processing mediated by left prefrontal cortex is important for reading. (iii) These results suggest that training in rapid auditory discrimination can lead to the development of brain activation in response to rapid stimuli in the left prefrontal cortex of the adult brain. The dyslexic participants in this study had both language deficits and rapid auditory processing deficits (as demonstrated by their performance on the three-tone identification test), and further studies will be required to determine how representative these results are to other groups of dyslexic adults.
Given the auditory nature of the task, it may be unexpected that frontal brain areas were sensitive to the rapidity of acoustic stimuli rather than auditory regions in temporal cortex. However, extensive connections have been demonstrated in primates (42) between auditory regions in the temporal cortex and the dorsolateral frontal regions homologous to those identified in the present study. There is, furthermore, convergent evidence that left prefrontal areas are involved in rapid auditory processing. A PET study (34) with stimuli similar to those used in our study showed activation in the same left prefrontal region for rapid stimuli relative to a resting baseline, but no activation for slow stimuli relative to the same baseline (the difference between rapid and slow stimuli was not statistically significant at the threshold used in that study). Whether the difference between rapid and slow stimuli exceeds threshold may be accounted for by different imaging methodologies (PET vs. fMRI), different task demands (passive presentation vs. pitch judgment), differences in stimuli, or different analyses. Further support comes from work that indicates this region shows increased activity as linguistic acoustic stimuli are presented with increased rapidity (R.A.P., E.T., A.P., S.N., P.T., M.M.M., and J.D.E.G., unpublished results). In that study, auditory sentences were compressed to various degrees, increasing the rapidity of the acoustic transitions in the speech stream. A similarly localized left prefrontal cortex increased in activity as compression increased. Another PET study (43) showed that when linguistic and nonlinguistic stimuli were grouped according to the presence or absence of rapidly changing acoustic cues, a region of the left frontal cortex was differentially active for the stimuli with rapidly changing acoustic cues. The region identified in that case was more posterior than observed in this study, which may be due to differences in task, stimuli, and perhaps imaging methodology.
In addition to the differences between normal and dyslexic readers in left prefrontal cortex processing of rapid stimuli, there were differences in right cerebellar response to rapid stimuli. Right cerebellar hemisphere and left frontal cortex are connected anatomically and have been shown to activate coincidentally (44, 45). However, the role of the cerebellum in language processing is not currently known (44). Other studies have also suggested that the cerebellum may be dysfunctional in dyslexia. Behavioral research has shown that dyslexics have deficits on tasks that rely on cerebellar integrity (46). In addition, a PET study (47) of motor sequence learning in dyslexic adults found that control subjects had more activity than dyslexics did in right cerebellum for prelearned and new motor sequences as compared with rest. Of particular interest is the role that has been demonstrated for the cerebellum in timing mechanisms for both motor and cognitive processes (44). Although it is possible that the processing of rapidly changing stimuli involves timing mechanisms that require the cerebellum, additional research is necessary to establish this connection.
There were important differences in the observed group difference in cerebellar and left prefrontal response to rapid stimuli. For the left prefrontal cortex, normal readers exhibited increased activity during rapid as compared with slow stimuli, whereas dyslexic readers exhibited no differential response (both stimuli elicited approximately equal activity). The right cerebellum, on the other hand, was more active for rapid as compared with slow stimuli in normal readers but showed the opposite pattern in dyslexic readers and was more active for slow as compared with rapid stimuli. Additionally, whereas the differential response to rapid as compared with slow stimuli was correlated with the subjects' rapid auditory processing performance in the left frontal cortex, it was not correlated in the right cerebellum. Another important difference between the two regions' disruptions in dyslexic readers is in their response to training. The lack of left frontal responsiveness was at least partially ameliorated by training; whereas (at least in the three subjects in this study) training did not influence the abnormal cerebellar response.
The processing of transient acoustic signals has long been proposed as an important element of language ability and an underlying factor in language-based learning disabilities. There has, however, been little direct evidence about what brain systems support such processing. The present findings and those of Belin et al. (34) point to the role of the left prefrontal region in processing of nonlinguistic rapidly changing acoustic stimuli. The absence of left prefrontal activation in dyslexic readers and the growth of such activation in response to training provide convergent evidence for an important role of this area in language comprehension and reading. These results suggest that functional brain imaging not only may reveal the brain systems that mediate rapid auditory processing but also may contribute to diagnosis and treatment of reading disorders by visualizing atypical responses and brain plasticity in response to remediation.
Acknowledgments
We thank Yvette Marquez, Justin Ream, and Moriah Thomason for assistance in subject recruitment and testing. This work was supported by grants from the Howard Hughes Medical Foundation (E.T.), Alcohol, Drug Abuse, and Mental Health Administration's Institute on Aging (J.D.E.G.), and McDonnell-Pew Program in Cognitive Neuroscience (R.A.P.).
Abbreviations
- fMRI
functional MRI
- PET
positron emission tomography
- ISI
interstimulus interval
- ROI
region of interest
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240461697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240461697
References
- 1.Shaywitz S E. N Engl J Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- 2.Pennington B F, Van Orden G C, Smith S D, Green P A, Haith M M. Child Dev. 1990;61:1753–1778. [PubMed] [Google Scholar]
- 3.Galaburda A M, Sherman G F, Rosen G D, Aboitiz F, Geschwind N. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 4.Rumsey J M, Andreason P, Zametkin A J, Aquino T, King A C, Hamburger S D, Pikus A, Rapoport J L, Cohen R M. Arch Neurol. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- 5.Hagman J O, Wood F, Buchsbaum M S, Tallal P. Arch Neurol. 1992;49:734–739. doi: 10.1001/archneur.1992.00530310082015. [DOI] [PubMed] [Google Scholar]
- 6.Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak R S, Frith C D. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Rumsey J M, Nace K, Donohue B, Wise D, Maisog J M, Andreason P. Arch Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- 8.Brunswick N, McCrory E, Price C J, Frith C D, Frith U. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- 9.Eden G F, VanMeter J W, Rumsey J M, Maisog J M, Woods R P, Zeffiro T A. Nature (London) 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- 10.Demb J B, Boynton G M, Heeger D J. Proc Natl Acad Sci USA. 1997;94:13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaywitz S E, Shaywitz B A, Pugh K R, Fulbright R K, Constable R T, Mencl W E, Shankweiler D P, Liberman A M, Skudlarski P, Fletcher J M, et al. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus N, McGee T J, Carrell T D, Zecker S G, Nicol T G, Koch D B. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich M M. Proc Natl Acad Sci USA. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Ann Neurol. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- 15.Pennington B F, Gilger J W, Pauls D, Smith S A, Smith S D, DeFries J C. J Am Med Assoc. 1991;266:1527–1534. [PubMed] [Google Scholar]
- 16.Cornelissen P L, Hansen P C, Hutton J L, Evangelinou V, Stein J F. Vision Res. 1998;38:471–482. doi: 10.1016/s0042-6989(97)00199-5. [DOI] [PubMed] [Google Scholar]
- 17.Livingstone M S, Rosen G D, Drislane F W, Galaburda A M. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovegrove W J, Bowling A, Badcock D, Blackwood M. Science. 1980;210:439–440. doi: 10.1126/science.7433985. [DOI] [PubMed] [Google Scholar]
- 19.Kuhl P K. In: Mechanistic Relationships Between Development And Learning. Carew T J, Menzel R, Shatz C J, editors. New York: Wiley; 1998. pp. 53–73. [Google Scholar]
- 20.Carr T, Posner M I. In: Language and Literacy: Comparative Approaches. DeGelder B, Morais J, editors. Cambridge, MA.: MIT Press; 1995. pp. 267–294. [Google Scholar]
- 21.Wagner R K, Torgesen J K. Psychol Bull. 1987;101:192–212. [Google Scholar]
- 22.Tallal P. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 23.Merzenich M M, Jenkins W M, Johnston P, Schreiner C, Miller S L, Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 24.Tallal P, Miller S L, Bedi G, Byma G, Wang X, Nagarajan S S, Schreiner C, Jenkins W M, Merzenich M M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 25.Tallal P, Galaburda A M, Llinás R R, von Euler C. Temporal Information Processing in the Nervous System. New York: Annals of the New York Academy of Sciences; 1993. [Google Scholar]
- 26.Farmer M E, Klein R M. Psychol Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- 27.Helenius P, Uutela K, Hari R. Brain. 1999;122:907–913. doi: 10.1093/brain/122.5.907. [DOI] [PubMed] [Google Scholar]
- 28.McAnally K I, Stein J F. Proc R Soc London Ser B. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- 29.Witton C, Talcott J B, Hansen P C, Richardson A J, Griffiths T D, Rees A, Stein J F, Green G G. Curr Biol. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- 30.Adlard A, Hazan V. Q J Exp Psychol A. 1998;51:153–177. doi: 10.1080/713755750. [DOI] [PubMed] [Google Scholar]
- 31.Schulte-Korne G, Deimel W, Bartling J, Remschmidt H. Neurosci Lett. 1999;276:41–44. doi: 10.1016/s0304-3940(99)00785-5. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz B, Rumsey J M, Donohue B C. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli J D, Moseley M E, Poldrack R A. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 34.Belin P, Zilbovicius M, Crozier S, Thivard L, Fontaine A, Masure M C, Samson Y. J Cognit Neurosci. 1998;10:536–540. doi: 10.1162/089892998562834. [DOI] [PubMed] [Google Scholar]
- 35.Glover G H, Lai S. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 36.Bandettini P A, Jesmanowicz A, Wong E C, Hyde J S. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 37.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Friston K J, Holmes A P, Price C J, Buchel C, Worsley K J. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 39.Merzenich, M. M., Miller, S. L., Protopapas, A., Saunders, G., Peterson, B. & Tallal, P. (2000) J. Learn Disab., in press.
- 40.Annett M. Q J Exp Psychol. 1967;19:327–333. doi: 10.1080/14640746708400109. [DOI] [PubMed] [Google Scholar]
- 41.Baldeweg T, Richardson A, Watkins S, Foale C, Gruzelier J. Ann Neurol. 1999;45:495–503. doi: 10.1002/1531-8249(199904)45:4<495::aid-ana11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Romanski L M, Bates J F, Goldman-Rakic P S. J Comp Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 43.Fiez J A, Raichle M E, Miezin F M, Petersen S E, Tallal P, Katz W F. J Cognit Neurosci. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- 44.Ivry R B, Fiez J A. In: The New Cognitive Neurosciences. 2nd Ed. Gazzaniga M S, editor. Cambridge, MA: MIT Press; 1999. pp. 999–1011. [Google Scholar]
- 45.Desmond J E, Gabrieli J D E, Glover G H. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- 46.Fawcett A J, Nicolson R I, Dean P. Ann Dyslexia. 1996;46:259–283. doi: 10.1007/BF02648179. [DOI] [PubMed] [Google Scholar]
- 47.Nicolson R I, Fawcett A J, Berry E L, Jenkins I H, Dean P, Brooks D J. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]