Abstract
Objectives: To determine whether patients with treated depression but no other risk factors for coronary heart disease (CHD) have abnormal arterial endothelial function, an abnormality that is common to other acquired risk factors for CHD.
Design: Case–control study.
Setting: Secondary care departments of cardiology and psychiatry in a single centre and the surrounding community.
Participants: Patients with treated depression and matched healthy controls, aged 18–55 years, without conventional acquired risk factors for CHD. These were recruited from local community mental health clinics, general practices, and patient support groups, and through posters placed in public areas of the hospital. Patients had major depression as defined in the American Psychiatric Association’s Diagnostic and statistical manual of mental disorders, fourth edition. Fifteen patients and 12 controls were recruited, and 12 patients and 10 controls completed the study.
Outcomes: Brachial artery flow mediated dilatation and baroreflex sensitivity.
Results: Arterial endothelial function measured by flow mediated dilatation was impaired in depression (mean (SEM) −0.7% (1.7%)) compared with controls (5.7% (0.9%), p = 0.005 by non-paired t test). Baroreflex sensitivity did not differ significantly between the groups.
Conclusion: Arterial endothelial function is impaired in treated depression. This abnormality may contribute to the increased risk of CHD seen in depression.
Keywords: endothelial function, depression, coronary heart disease, baroreceptor sensitivity
There is increasing epidemiological evidence that depression is a risk factor for the development of coronary heart disease (CHD). While some conventional risk factors (for example, smoking) frequently coexist with depression, since 1993 at least seven published cohort studies and one case–control study have controlled for conventional risk factors and yet have still shown convincingly that depression is an important independent risk factor for CHD in populations without CHD at the outset.1–8 Overall, these studies indicate an approximately twofold increased risk.
Several mechanisms have been proposed by which depression may predispose to CHD. These include impaired arterial endothelial function,9 hypothalamic-pituitary-adrenal axis overactivity with related increased activity of the sympathetic nervous system,10 increased platelet aggregability,11 and abnormal folate and homocysteine metabolism.12 We examined the first of these possibilities in a group of patients with treated major depression and in a group of age and sex matched controls. Arterial endothelial function was assessed by measurement of brachial artery flow mediated dilatation (FMD). We also measured baroreflex sensitivity (BRS), a measure of autonomic control of heart rate in response to changes in blood pressure, which is reduced when sympathetic nervous system activity is increased.
METHOD
Ethical permission for this study was given by our local research ethics committee. Patients receiving treatment for major depression were recruited from local community mental health clinics, general practices, and patient support groups. All were evaluated by a psychiatrist and fulfilled the diagnostic criteria for primary major depression as described in the Diagnostic and statistical manual of mental disorders, fourth edition, of the American Psychiatric Association. All were considered to be in remission at the time they were studied, based on a subjective assessment of their current symptoms, their ability to function socially, and that they had remained stable on their current medications for a minimum of three months. Controls were recruited by poster from hospital staff and students and from relatives of cardiology outpatients and inpatients. Exclusion criteria for both groups were a history of heart disease or any acquired risk factors for CHD (smoking within the previous 10 years, hypertension, diabetes, hypercholesterolaemia, and hyperhomocysteinaemia). Other exclusion criteria were body mass index greater than 30, current pregnancy or menopause, or any other condition, drug treatment, or dietary supplements known or likely to affect the measured variables. All subjects were in the age range 18–55 years. Patients were studied on their prescribed antidepressants (table 1).
Table 1.
Antidepressants prescribed to the patient group
| Subject | Antidepressant(s) | Type |
| 1 | Phenelzine | MAOI |
| Lithium | ||
| 2 | Fluoxetine | SSRI |
| Lithium | ||
| 3 | Reboxetine | NARI |
| Mirtazipine | PARA | |
| 4 | Venlafaxine | SNRI |
| Trazodone | TCA | |
| 5 | Sertraline | SSRI |
| 6 | Sertraline | SSRI |
| 7 | Fluoxetine | SSRI |
| 8 | Paroxetine | SSRI |
| 9 | Citalopram | SSRI |
| 10 | Venlafaxine | SNRI |
| 11 | None (non-compliant) | |
| 12 | None (non-compliant) |
MAOI, monoamine oxidase inhibitor; NARI, noradrenaline (norepinephrine) reuptake inhibitor; PARA, presynaptic α2 receptor antagonist; SNRI, serotonin and noradrenaline reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
All participants were studied in the morning beginning at 8 30 am, fasted, and having avoided alcohol for at least 24 hours and caffeine for at least 12 hours. After venesection, attachment to an ECG monitor and fitting of a Portapres (TNO Biomedical Instrumentation, Affligem, Belgium) non-invasive continuous blood pressure monitor, subjects rested supine in a quiet, temperature controlled environment for 20 minutes. For the next 10 minutes their heart rate and blood pressure were recorded. Analogue output from the ECG monitor and the Portapres were fed into an analogue to digital converter (Biopac, Biopac Systems Inc, Goleta, California, USA) and output from the converter was fed into a PC and recorded at 500 Hz using AcqKnowledge software (Biopac Systems Inc) for later calculation of BRS by the “sequence” method, as previously described.13 Briefly, each systolic blood pressure measurement was matched with the RR interval during which it occurred. A proprietary program was then used to seek “sequences” in which both systolic pressure and RR interval increased or decreased together for three or more beats. Minimum increments were 1 mm Hg and 2 ms. A second program selected sequences in which systolic blood pressure and RR interval had a correlation coefficient of 0.85 or higher. The mean gradient of these sequences, expressed in ms/mm Hg, was taken as the BRS.
On completion of the recording of heart rate and blood pressure, FMD of the brachial artery was measured using a technique previously validated in our department.14 Briefly, high resolution ultrasound (7.5 MHz) was used to visualise the brachial artery above its bifurcation at the elbow. An M mode of the ultrasound image was fed to a wall tracking system (Vadirec 101, Medical Systems Arnhem, Oosterbrek, Netherlands), which can determine the diastolic diameter of the artery to within 10 μm. A wrist cuff was then inflated to suprasystolic pressure for five minutes and released, causing reactive hand hyperaemia, which in turn led to increased flow through the observed segment of the brachial artery. Increased flow through an artery increases the shear stress at the blood endothelium interface and stimulates healthy endothelial cells to produce the vasodilator nitric oxide. Some of the nitric oxide produced diffuses into the vascular smooth muscle layer adjacent to the endothelium, inducing relaxation and vessel dilatation. The dilatation of the brachial artery in response to increased flow—the “flow-mediated dilatation”—was measured at one minute intervals for the next eight minutes. The greatest change from baseline within the first three minutes was taken to be FMD and was expressed as the percentage of the baseline diameter. Once the artery had returned to baseline diameter, 400 μg of glyceryl trinitrate was administered sublingually and brachial artery dilatation was measured three minutes later as a measure of endothelium independent dilatation.
RESULTS
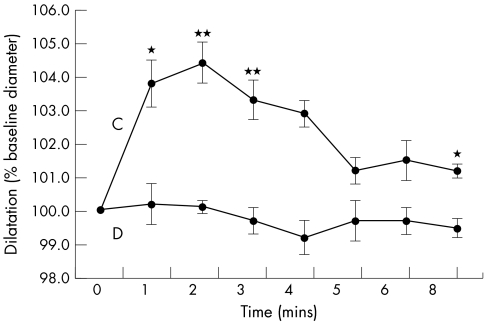
Five subjects were excluded after enrolment. Two patients with depression and one control subject were found to have hypercholesterolaemia, one control subject was found to have hyperhomocystinaemia, and FMD could not be calculated for one patient with depression because of movement artefact. Twelve patients with depression and 10 controls completed the study. The two groups were well matched for age, body mass index, cholesterol, folate, and homocysteine (table 2). In the depression group 50% were men but in the control group 80% were men (difference not significant by χ2 test). Student’s unpaired t test was used to compare measurements between groups and data are expressed as mean (SEM). Supine blood pressure, resting heart rate, and BRS did not differ significantly. Mean baseline brachial artery diameter was similar in both groups. FMD was significantly impaired in patients with depression (−0.7% (1.7) v 5.7% (0.9), p = 0.005). Glyceryl trinitrate induced endothelium independent dilatation of the brachial artery was similar in patients and controls (18.5% (2.4) v 19.0% (2.5), NS; table 3). Figure 1 illustrates the time course of brachial artery diameter changes for each group.
Table 2.
Characteristics of depressed and control study groups
| Depression | Control | |
| Number | 12 (6 male) | 10 (8 male) |
| Age (years) | 39, range 24–51 | 35, range 24–50 |
| Body mass index | 26 (0.6) | 25 (1.0) |
| Total cholesterol (mmol/l) | 4.9 (0.3) | 5.0 (0.3) |
| Folate (nmol/l) | 10.5 (1.6) | 9.3 (1.1) |
| Homocysteine (μmol/l) | 9.4 (0.8) | 9.3 (0.9) |
Data are mean (SEM).
Table 3.
Results of tests in depressed and control groups
| Depression | Control | p Value (non-paired t test) | |
| Systolic BP (mm Hg) | 117 (2.7) | 113 (3.6) | NS |
| Diastolic BP (mm Hg) | 76 (5.2) | 71 (2.8) | NS |
| BRS (ms/mm Hg) | 19.3 (2.8) | 20.9 (2.6) | NS |
| BA baseline diameter (mm) | 3.44 (0.23) | 3.38 (0.22) | NS |
| FMD (%) | −0.7 (1.7) | 5.7 (0.9) | 0.005 |
| GTN dilatation (%) | 18.5 (2.4) | 19.0 (2.5) | NS |
Data are mean (SEM).
BA, brachial artery; BP, blood pressure; BRS, baroreceptor sensitivity; FMD, flow mediated dilatation; GTN, glyceryl trinitrate.
Figure 1.
Time course of brachial artery dilatation following cuff release. C, control group; D, depression group.*p<0.05; **p<0.01.
DISCUSSION
The principal finding of this study was that arterial endothelial function, as measured by FMD of the brachial artery, is significantly impaired in patients with treated depression. Our finding agrees with that of Rajagopalan and colleagues,9 who recently showed that FMD is impaired in untreated depression, and suggests that the abnormality persists despite a satisfactory clinical response to antidepressants.9 Reduced or absent brachial artery FMD is common to all acquired conditions that are established major risk factors for CHD, namely, smoking, hypertension, diabetes, and hypercholesterolaemia. It is considered to indicate decreased bioavailability of endothelial nitric oxide, because of either decreased production and release or increased inactivation, an abnormality that is atherogenic.15 The cause of impaired FMD in depression is not known but, drawing parallels with the established risk factors mentioned above, this abnormality may be an important mechanism by which depression increases the risk of CHD.
The second finding was that BRS did not differ between the depression and control groups.
In patients with established CHD, the presence of depression was associated with a lower BRS.16 Furthermore, low BRS is associated with increased mortality (particularly from sudden cardiac death) following myocardial infarction.17 The increased risk associated with lower BRS is thought to be mediated by increased sympathetic activity. We hypothesised that reduced BRS may be present in depressed patients without overt CHD caused by increased sympathetic nervous activity, which may provide an additional mechanism for increased risk of developing CHD (through increased blood pressure, heart rate, platelet aggregation, etc). Our findings do not support this hypothesis. However, given the wide variability in BRS in both depression and control groups and the small number of subjects, the power of this study to detect a difference between our two groups was low. In addition, by excluding patients with hypertension we may have screened out some of those with the greatest hypothesised increased sympathetic nervous system activity.
There is evidence that the selective serotonin reuptake inhibitor paroxetine may impair endothelial nitric oxide synthase, the enzyme that produces nitric oxide, and therefore endothelial function.18,19 In our study only one subject was being treated with paroxetine and this person had an FMD greater than the depression group average. In total, six subjects with depression were taking selective serotonin reuptake inhibitor alone or in combination and their mean FMD (−1.2%) did not differ significantly from that of the remaining six in the group (−0.2%).
Given that impaired endothelial function is present in untreated depression, there are at least two possible reasons that may explain its persistence in our subjects despite effective drug treatment. The first is that impaired endothelial function is a “trait” rather than a “state” phenomenon, associated somehow with proneness to depression itself, and remains present, to a greater or lesser extent, despite a significant improvement in symptoms. The second is that the antidepressants taken by our subjects are responsible for the impaired endothelial function observed, either by causing it directly or by preventing its resolution as symptoms improve.
Unipolar depression was considered by the World Health Organization to be the leading cause of disability worldwide in 1990.20 CHD is already the leading cause of death in the developed world and the WHO projects it to be the leading cause of death worldwide by 2020. It is known that these two diseases are closely associated, in particular that those with clinically manifest CHD are much more likely than those without to suffer from depression and that, other risk factors being equal, depression is associated with increased mortality in this same group.21 There is also growing epidemiological evidence that depression is a primary risk factor for the development of CHD. Depression is characterised by overactivity of the hypothalamic-pituitary-adrenal axis. Enlargement of the pituitary and adrenal glands, hypercortisolaemia, and failure of cortisol to be suppressed following dexamethasone administration have all been reported.10 This implies that the arterial endothelium of patients with depression is exposed to generally raised and poorly suppressible concentrations of cortisol. Recent evidence has shown that oral cortisol treatment for five days impairs endothelial function in normal subjects.22 The observed impairment of endothelial function in depression may therefore be caused by chronically raised cortisol concentrations, which are due in turn to hypothalamic-pituitary-adrenal axis overactivity. Interestingly, acute mental stress has recently been shown to impair endothelial function in healthy subjects.23 Although the mechanism of this effect has not been investigated, the cortisol response to mental stress is a plausible explanation. It is a limitation of our study that we did not measure cortisol. In addition, we cannot exclude the possibility that our observation is due in part to a heterogeneous antidepressant drug effect.
The importance of this finding is that effective treatment of depression with current antidepressants was associated with impaired endothelial function in a group of subjects who were typical of the wider population of patients with treated depression. Whether this abnormality was caused by the disease, secondary to drug treatment, or caused by a combination of both, its very presence may contribute to such patients’ increased risk of developing CHD.
Supplementary Material
Abbreviations
BRS, baroreflex sensitivity
CHD, coronary heart disease
FMD, flow mediated dilatation
REFERENCES
- 1.Anda R, Williamson D, Jones D, et al. Depressed affect, hopelessness, and the risk of ischaemic heart disease in a cohort of US adults. Epidemiology 1993;4:285–94. [DOI] [PubMed] [Google Scholar]
- 2.Vogt T, Pope C, Mullooly J, et al. Mental health status as a predictor of morbidity and mortality: a 15-year follow-up of members of a health maintenance organization. Am J Public Health 1994;84:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation 1996;93:1976–80. [DOI] [PubMed] [Google Scholar]
- 4.Pratt LA, Ford DE, Crum RM, et al. Depression, psychotropic medication, and risk of myocardial infarction: prospective data from the Baltimore ECA follow-up. Circulation 1996;94:3123–9. [DOI] [PubMed] [Google Scholar]
- 5.Hippesley-Cox J, Fielding K, Pringle M. Depression as a risk factor for ischaemic heart disease in men: a population based case-control study. BMJ 1998;316:1714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford DE, Mead LA, Chang PP, et al. Depression is a risk factor for coronary artery disease in men. Arch Intern Med 1998;158:1422–6. [DOI] [PubMed] [Google Scholar]
- 7.Ferketich AK, Schwartzbaum JA, Frid DJ, et al. Depression as an antecedent to heart disease among women and men in the NHANES I study. Arch Intern Med 2000;160:1261–8. [DOI] [PubMed] [Google Scholar]
- 8.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular health study collaborative research group. Circulation 2000;102:1773–9. [DOI] [PubMed] [Google Scholar]
- 9.Rajagopalan S, Brook R, Rubenfire M, et al. Abnormal brachial artery flow-mediated vasodilatation in young adults with major depression. Am J Cardiol 2001;88:196–8. [DOI] [PubMed] [Google Scholar]
- 10.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry 1998;55:580–92. [DOI] [PubMed] [Google Scholar]
- 11.Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996;153:1313–7. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey PSA, Toone BK, Carney MW, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet 1990;336:392–5. [DOI] [PubMed] [Google Scholar]
- 13.Parlow J, Viale J, Annat G, et al. Spontaneous cardiac baroreflex in humans: comparison with drug-induced responses. Hypertension 1995;25:1058–68. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey MW, Goodfellow J, Jones CJ, et al. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation. 1995;92:3212–9. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995;96:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J 1999;137:453–7. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Bigger JT, Marcus FI, et al for the ATRAMI investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–84. [DOI] [PubMed] [Google Scholar]
- 18.Shimbo DS, Geer E, Child J, et al. The selective serotonin reuptake inhibitor paroxetine inhibits endothelial-dependent vasodilatation. J Am Coll Cardiol 2000;35(2 suppl A):271.10676669 [Google Scholar]
- 19.Finkel MS, Laghrisi-Thode F, Pollock BG, et al. Paroxetine is a novel nitric oxide synthase inhibitor. Psychopharmacol Bull 1996;32:653–8. [PubMed] [Google Scholar]
- 20.World Health Organization. The world health report: 1999: making a difference. Geneva: WHO, 1999.
- 21.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999–1005. [DOI] [PubMed] [Google Scholar]
- 22.Mangos GJ, Walker BR, Kelly JJ, et al. Cortisol inhibits cholinergic vasodilation in the human forearm. Am J Hypertens 2000;13:1155–60. [DOI] [PubMed] [Google Scholar]
- 23.Ghiadoni L, Donald A E, Cropley M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation 2000;102:2473–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.