Abstract
Objective: To investigate matrix metalloproteinases (MMP-2 and MMP-9) in heart failure caused by ischaemic and idiopathic dilated cardiomyopathy, and the impact of angiotensin converting enzyme (ACE) inhibition on MMP.
Design and main outcome measures: MMP were extracted from myocardium of patients with heart failure (coronary artery disease, n = 13; idiopathic dilated cardiomyopathy (IDCM), n = 16) and from controls (n = 6). The active form of MMP-2 and MMP-9 was measured by enzyme linked immunosorbent assay; activity of MMPs by zymography; mRNA expression of MMPs by reverse transcriptase polymerase chain reaction.
Results: Active MMP-9 was significantly increased in coronary artery disease (mean (SD) 1.6 (0.35) ng/ml) and IDCM (2.11 (0.54) ng/ml) in comparison with controls (0.53 (0.15) ng/ml). Increased MMP-2 was only found in IDCM (3.68 (0.41) ng/ml). There were corresponding increases in MMP activity but no upregulation of mRNA expression was found. The ACE inhibitors captopril and ramiprilate inhibited MMP-2 and MMP-9 activity in vitro (inhibitory capacity (IC50), in mmol/l: MMP-2: captopril 2.0 (0.16), ramiprilate 2.1 (0.3); MMP-9: captopril 1.65 (0.18), ramiprilate 2.0 (0.3)). Lisinopril inhibited MMP-9 significantly but did not inhibit MMP-2 in vitro (IC50 MMP-2: 7.4 (0.88); MMP-9: 7.86 (2.23)). Inhibition of MMP activity by ACE inhibitors was blunted by zinc excess.
Conclusions: Upregulation of MMP-9 activity is common in the failing myocardium, independent of the underlying disease. Missing upregulation of transcription suggests that activation of latent forms of MMP is the source of increased MMP activity, rather than increased de novo synthesis. Some ACE inhibitors may influence MMP activity by a direct effect.
Keywords: remodelling, matrix metalloproteinase, heart failure, angiotensin converting enzyme inhibitor
Recent results from the ELITE-2 (evaluation of losartan in the elderly) study,1 which was designed to show the superiority of losartan over captopril, failed even to give an assurance that these two agents are of equivalent benefit in heart failure treatment. This result has led to a re-examination of the factors that determine the actions of angiotensin converting enzyme (ACE) inhibitors and sartans in the failing heart. ACE inhibitors inhibit angiotensin converting enzyme, which catalyses the formation of angiotensin II from angiotensin I; sartans are thought to inhibit the ACE system more effectively by angiotensin II receptor antagonism. On the other hand, the haemodynamic consequences of ACE inhibition and angiotensin II receptor antagonism only partially explain their beneficial effects on cardiac remodelling in heart failure.2 Local mechanisms have been suggested—for example, inhibition of the tissue ACE system and a decrease in the breakdown of bradykinin.3 Sorbi and colleagues4 suggested that reduced proteinuria in hypertensive patients treated with captopril reflected a direct effect of captopril on matrix metalloproteinase-2 (MMP-2) and MMP-9 activity, mediated by the sulfydryl group on the ACE inhibitor.
Comparing the effect of ACE and MMP inhibition on cardiac remodelling in a rapid pacing induced heart failure model in pigs, McElmurray and colleagues showed that both MMP and ACE inhibitors significantly reduced the degree of left ventricular dilatation.5 However, MMP activity in the left ventricular myocardium did not differ between animals treated with MMP inhibitors, ACE inhibitors, or combined treatment. So the question arises as to whether ACE inhibitors have a direct influence on myocardial MMP activity.
In the light of increasing interest in the role of the cardiac proteolytic system, our aim in this study was to investigate the gelatinases MMP-2 and MMP-9 in hearts that had failed because of coronary artery disease or idiopathic dilated cardiomyopathy (IDCM), and the influence of ACE inhibitors on these enzymes.
METHODS
Subjects
Human left ventricular heart tissue was obtained from explanted hearts at the time of heart transplantation. Samples of myocardium from 16 hearts with IDCM, 13 with ischaemic cardiomyopathy, and six brain dead organ donor hearts (which could not be used for heart transplantation) were snap frozen in liquid nitrogen after explantation and stored at −80°C until analysis. IDCM was diagnosed according to recent World Health Organization criteria.6 The severity of heart failure did not differ between the IDCM and coronary artery disease groups.
The protocol of the study was approved by the ethics commission at the University of Jena.
Preparation of MMP
Approximately 25 mg of frozen adult left ventricle was washed three or four times with cold saline. Cardiac tissue was then incubated in 1000 μl of extraction buffer (10 mmol/l cacodylic acid at pH 5, 0.15 mol/l NaCl, 1 μmol/l ZnCl2, 20 mmol CaCl2, 1.5 mmol/l NaN3, and 0.01% (vol/vol) Triton X 100) at 4°C for 72 hours. The extraction buffer was collected and the pH was raised by the addition of 1.5 mol/l Tris HCl buffer (pH 8.8). Aliquots from these samples were loaded directly onto substrate gels.
Total protein in extracts
A Bio-Rad dye binding assay (Palo Alto, California, USA) was used to estimate the total protein concentration in tissue extracts according to Bradford.7
Visualisation of gelatinolytic activities on SDS substrate gels (zymography)
Sodium dodecyl sulfate (SDS) substrate gels were prepared as published elsewhere,8 with modification.9 Gelatine (porcine skin, 300 bloom from Sigma, St Louis, Missouri, USA) was added to standard Laemmli acrylamide polymerisation mixture at a final concentration of 1 mg/ml. Tissue extract was mixed with substrate gel sample buffer (10% SDS, 0.25 mol/l Tris HCl pH 6.8, and 0.1% wt/vol bromphenol blue) to a final protein concentration of 1000 mg/ml, and 20 μl were loaded under non-reducing conditions immediately without boiling into wells of a 4% (wt/vol) acrylamide Laemmli stacking gel on a cast vertical gel. Gels were run at 15 mA/gel while stacking, and at 20 mA/Gel during the separation phase at room temperature. Following electrophoresis the gels were soaked in 2.5% (wt/vol) Triton X 100 with gentle shaking for 30 minutes at room temperature with one change of detergent solution. The gels were rinsed and incubated overnight at 37°C in substrate buffer (50 mmol/l Tris HCl pH 8, 5 mmol/l CaCl2, and 0.02% wt/vol NaN3).
After incubation, the gels were stained for three minutes in Coomassie blue R250 in acetic acid:isopropyl alcohol:water (1:3:6 by volume), and destained in water overnight with or without ACE inhibitors (captopril, lisinopril: Sigma; ramiprilate: Aventis, Bad Soden, Germany). Gels were scanned and analysed by densitometry for lysis band intensity (Herolab Imaging system, Wiesloch, Germany). The MMP gelatinolytic activity results are expressed in arbitrary units expressing the videodensitometrically determined ratio of the MMP to the activity of MMP purified standard proteins (Invitek, Berlin, Germany). All experiments were done in triplicate.
Measurement of active MMP
Measurement of active MMP-2 and MMP-9 in human tissue extracts and pleural and pericardial effusion was undertaken using commercially available enzyme linked immunosorbent assay (ELISA) kits (Amersham Life Science, Burlingame, USA).
Western blot
MMP protein was detected by monoclonal antibodies against the proenzyme and active MMP-2 and MMP-9 (Calbiochem, San Diego, California, USA), employing a standard western blot technique.8
RNA isolation: cDNA synthesis and quantitative RT-PCR
Total RNA from myocardial tissue was isolated according to the RNeasy protocol from Qiagen (Hilden, Germany) and converted to cDNA using a commercially available kit from Promega (Madison, Wisconsin, USA). The polymerase chain reaction (PCR) MIMIC construction kit from Clontech (Palo Alto, California, USA) was used to produce a DNA fragment of known size and amount, with primer binding sites for the housekeeping gene glyceraldehyde-3-phosphodehydrogenase (GAPDH) as previously reported.10 To normalise cDNA samples, a competitive PCR from the housekeeping gene GAPDH was undertaken using this competitor fragment, under conditions reported before.10 Separate reverse transcriptase polymerase chain reaction assays (RT-PCR) from 2 μl of the equilibrated cDNA were done using the GAPDH primer pair (forward: 5′AGC CAC ATC GCT CAG AAC AC; reverse 5′GAG GCA TTG CTG ATG ATC TTG, patent pending) and the primer specified in table 1. The resulting PCR products were separated on 2.0% agarose gel. The MMP mRNA results are given in arbitrary units expressing the videodensitometrically determined ratio of the MMP to the GAPDH RT-PCR products.
Table 1.
Primer used to detect mRNA expression by reverse transcriptase polymerase chain reaction
| Forward | Reverse | PCR product | TA | Accession code | |
| MMP-1 | AgCAAACACATCTgACCTAC | TAAAgAACATCACTTCTCCC | 564 | 55.3 | X 54925 |
| MMP-2 | AgTCTgCTCTgCCTATCCTC | ACACCCATATCTgTCTTCCC | 833 | 59.8 | NM00453 |
| MMP-3 | ACCCACTCTATCACTCACTCAC | CTgTTACTCTTCAAAgTgTgTgTC | 536 | 56.9 | J 03209 |
| MMP-9 | CCAgTTTCCATTCATCTTCC | ACAgTAgTggCCgTAgAAgg | 479 | 55.0 | NM004994 |
| CCCTTgTgCTCTTCCCTg (nested) | CCCgTCCTTCCCgTCgAAg (nested) | 303 | 60.0 | NM004994 | |
| TIMP-2 | gAAACgACATTTATggCAAC | gATgTTCTTCTCTgTgACCC | 377 | 58.3 | J 05593 |
MMP, matrix metalloproteinase; PCR, polymerase chain reaction; TA, annealing temperature; TIMP, tissue inhibitor of metalloproteinases.
Data analysis
Statistical analysis was done using Microsoft Excel data analysis software and SPSS (SPSS Inc, Chicago, Illinois, USA). Differences in the mean value were considered significant at a probability value of p < 0.05. Values are presented as mean (SD). Student’s t test was used to compare baseline characteristics between groups.
RESULTS
MMP-9 activity and mass in failing myocardium
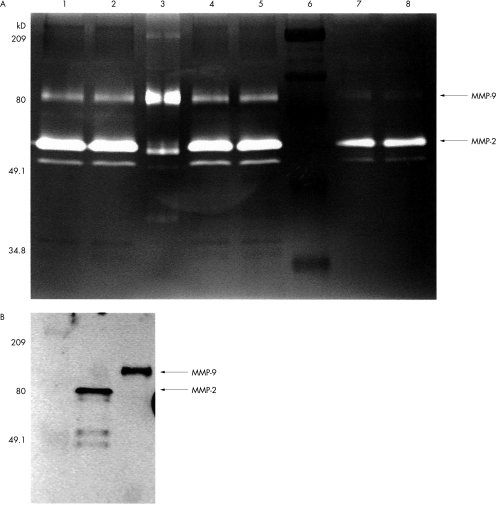
Human cardiac MMPs showed gelatinolytic activity in several bands on the overnight incubated, Coomassie stained, gelatine enriched SDS-PAGE gel. From the known molecular weights of the active forms of MMP-2 and MMP-9, the gelatinolytic bands of the purified proteins MMP-2 and MMP-9, and protein detection using Western blot analysis, active MMP-2 could be identified as a 66 kDa band and MMP-9 as an 86 kDa band at zymography (fig 1). Bands observed in some cases at 92 and 72 kDa should be regarded as gelatinolytic active enzymes, fragments, and proenzymes, partially activated by interaction with SDS-PAGE components.
Figure 1.
(A) Visualisation of gelatinolytic activities on sodium dodecyl sulphate (SDS) substrate gels (zymography). Extracts were loaded onto a SDS-PAGE gel containing 0.6 mg/ml gelatin under non-reducing conditions. Separation is followed by overnight incubation in substrate buffer, with and without angiotensin converting enzyme (ACE) inhibitor, and staining in Coomassie blue. Lanes 1 and 2, idiopathic dilated cardiomyopathy; lane 3, matrix metalloproteinase (MMP)-2 and MMP-9 purified standard proteins; lanes 4 and 5, coronary artery disease; lane 6, protein molecular weight standard (209, 80, 49.1, and 34.8 kDa bands are indicated); lanes 7 and 8, normal myocardium: arrows show the MMP-2 and MMP-9 bands. Inactive forms of MMP show gelatinolytic activity owing to alterations by SDS and during PAGE passage. (B) Western blot detection of MMP-2 and MMP-9.
Concentrations of active forms of MMP-9 measured by ELISA, as well as the gelatinolytic activity of MMP-9, were found to be increased in myocardium from patients with IDCM and coronary artery disease in comparison with the activity in non-failing hearts (table 2). The active form of MMP-2 and its gelatinolytic activity were increased in IDCM hearts, but there were no significant differences in MMP-2 determinations between non-failing and coronary diseased myocardium.
Table 2.
Concentration, activity, and mRNA expression of matrix metalloproteinases in idiopathic dilated cardiomyopathy and coronary artery disease
| MMP concentration (ELISA) | Zymographic activity of MMP | mRNA expression (RT-PCR) (MMP/ GAPDH in AU) | |||||||
| MMP-2 (ng/ml) | MMP-9 (ng/ml) | MMP-2 (AU) | MMP-9 (AU) | MMP-1/ GAPDH | MMP-2/ GAPDH | MMP-3/ GAPDH | MMP-9/ GAPDH | TIMP-2/ GAPDH | |
| IDCM (n=16) | 3.68 (0.41)* | 2.11 (0.54)* | 1196 (74)* | 802 (132)* | 0 | 0.492 | 0 | 0.22 | 1.16 |
| CAD (n=13) | 0.7 (0.26) | 1.6 (0.35)* | 571 (71) | 549 (71)* | 0 | 0.329 | 0 | <0.005† | 0.6 |
| Control (n=6) | 0.89 (0.35) | 0.53 (0.15) | 989 (158) | 245 (58) | 0.012 | 0.7 | 0 | 0.033 | 1.06 |
Values are mean (SD); mRNA expression is given in arbitrary units expressing the videodensitometrically determined ratio of MMP to GAPDH RT-PCR.
*p<0.05 v control.
†Nested reverse transcriptase polymerase chain reaction (RT-PCR).
AU, arbitrary units; CAD, coronary artery disease; ELISA, enzyme linked immunosorbent assay; GAPDH, glyceraldehyde-3-phosphodehydrogenase; IDCM, idiopathic dilated cardiomyopathy; MMP, matrix matalloproteinase; TIMP, tissue inhibitor of matrix metalloproteinases.
Source of increased MMP activity
Expression of MMP-2 mRNA measured by quantitative RT-PCR showed no significant differences between coronary artery disease, IDCM, and non-failing myocardium (table 3). With respect to MMP-9 expression, no increase could be detected in coronary artery disease and IDCM myocardium in comparison with non-failing myocardium. In coronary artery disease, MMP-9 mRNA expression was detectable only by a highly sensitive nested PCR.
Table 3.
Clinical and haemodynamic characteristics of patients with end stage heart failure from idiopathic dilated cardiomyopathy or coronary artery disease
| Patient number | Group | Age (years)/ sex | Weight (kg)/ height (cm) | NYHA class | Treatment | LVEF (%) | LVEDD (mm) | Creatinine (μmol/l) | CAD | MI |
| 1 | IDCM | 54/M | 70/180 | IV | ACEI, DIG, D, DO | 10 | 80 | 50 | – | – |
| 2 | IDCM | 60/M | 70/175 | IV | ACEI, BB, D, CA, DO | 18 | 72 | 110 | – | – |
| 3 | IDCM | 49/M | 90/188 | IV | ACEI, BB, D | 14 | 70 | 120 | – | – |
| 4 | IDCM | 41/M | 78/176 | III | ACEI, DIG, D, DO | 18 | 85 | 95 | – | – |
| 5 | IDCM | 68/M | 87/188 | IV | ACEI, BB, DIG, D | 15 | 68 | 105 | – | – |
| 6 | IDCM | 59/M | 91/196 | III | AT1, DIG, D, DO | 15 | 68 | 110 | – | – |
| 7 | IDCM | 41/M | 82/176 | IV | AT1, DIG, SPIRO, D | 20 | 72 | 130 | – | – |
| 8 | IDCM | 42/M | 105/186 | IV | ACEI, BB, DIG, D | 29 | 69 | 100 | – | – |
| 9 | IDCM | 46/M | 81/186 | III | ACEI, DIG, D | 17 | 71 | 96 | – | – |
| 10 | IDCM | 34/M | 73/178 | III | AT1, BB, D | 20 | 54 | 86 | – | – |
| 11 | IDCM | 70/M | 80/169 | III | AT1, BB, D | 34 | 69 | 170 | – | – |
| 12 | IDCM | 55/M | 81/175 | III | BB, D | 20 | 73 | 130 | – | – |
| 13 | IDCM | 66/M | 75/176 | IV | AT1, D, DO | 22 | 70 | 68 | – | – |
| 14 | IDCM | 45/M | 80/180 | IV | BB, D | 17 | 81 | 79 | – | – |
| 15 | IDCM | 64/M | 92/178 | III | ACEI, BB, DO | 22 | 77 | 135 | – | – |
| 16 | IDCM | 68/M | 75/169 | IV | AT1, BB, DO | 12 | 78 | 65 | – | – |
| 1 | CAD | 54/M | 78/172 | IV | AT1, D, DO | 23 | 72 | 82 | 1V | A |
| 2 | CAD | 53/M | 78/176 | III | ACEI, BB, DIG, D | 28 | 88 | 100 | 2V | P |
| 3 | CAD | 68/M | 86/178 | III | ACEI, D | 24 | 84 | 120 | 3V | A,P |
| 4 | CAD | 61/M | 91/169 | IV | ACEI, DIG, D, DO | 25 | 66 | 140 | 3V | A |
| 5 | CAD | 60/M | 58/170 | IV | ACEI, DIG, D, DO | 24 | 75 | 60 | 3V | A, P |
| 6 | CAD | 58/M | 81/190 | IV | ACEI, AT1, BB, DIG, D | 15 | 75 | 180 | 1V | P |
| 7 | CAD | 60/M | 89/188 | III | ACEI, BB, DIG, D | 25 | 82 | 160 | 2V | A, P |
| 8 | CAD | 58/M | 82/188 | III | ACEI, BB, DIG, D | 29 | 75 | 150 | 3V | P, A |
| 9 | CAD | 67/M | 80/178 | III | ACEI, AT1, D | 20 | 83 | 100 | 2V | P |
| 10 | CAD | 59/M | 79/175 | IV | AT1, DIG, D, DO | 33 | 64 | 76 | 3V | A |
| 11 | CAD | 57/M | 95/181 | III | BB, DIG, D | 23 | 85 | 100 | 2V | A |
| 12 | CAD | 52/M | 81/178 | IV | ACEI, D, DO | 10 | 88 | 90 | 3V | A, P |
| 13 | CAD | 40/M | 88/175 | IV | BB, SPIRO, DO | 18 | 78 | 72 | 3V | P |
A, anterior myocardial infarction; ACEI, angiotensin converting enzyme inhibitor; AT1, angiotensin II type 1 receptor antagonist; BB, β blocker; CA, calcium channel blocker; CAD, coronary artery disease; D, diuretics; DIG, digitalis glycosides; DO, dopamine or dobutamine; IDCM , idiopathic dilated cardiomyopathy; LVEDD, left ventricular end diastolic diameter assessed by echocardiography; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association functional class; P, posterior myocardial infarction; 1–3V, one, two, or three vessel coronary artery disease.
Except for the expression of MMP-1 in one patient with IDCM, we could not detect MMP-1 or MMP-3 expression in any of the diseased myocardium samples or in non-failing myocardium. TIMP-2 was highly expressed in all groups, with a tendency to increased expression in the diseased hearts. This did not reach significance because of the highly scattered data in both the IDCM and the coronary artery disease groups.
Effects of captopril, lisinopril, and ramiprilate on human MMP-9 in vitro
The inhibitory capacity of each ACE inhibitor on the MMPs was titrated. Figure 2 shows a representative example of dose dependent inhibition of zymographic activity by captopril. To compare the inhibitory effects of different ACE inhibitors on MMP-2 and MMP-9 in vitro, we titrated MMP inhibition of each ACE inhibitor until the gelatinolytic activity in zymography gel had disappeared. Untreated controls were regarded as having 100% activity. Table 4 shows the ACE inhibitor concentration that inhibits 50% of the gelatinolytic activity (IC50). Owing to the methodological characteristics of zymography and the fact that we used heart extracts containing several gelatinolytic enzymes, fragments, and activated proenzymes rather than purified enzymes, the IC50 value is only approximate. Captopril and ramiprilate significantly inhibited MMP-2 and MMP-9 activity in vitro, while lisinopril inhibited MMP-9 but not MMP-2 (table 4). Inhibition of MMP activity was blunted by excess zinc.
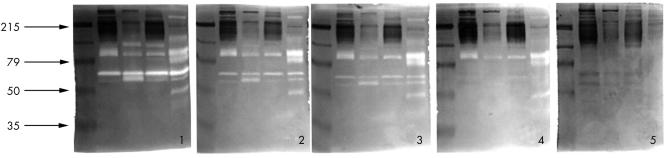
Figure 2.
Dose dependent inhibition of matrix metalloproteinases by the angiotensin converting enzyme (ACE) inhibitor captopril in vitro: gel 1, overnight incubation without ACE inhibitor; gel 2, overnight incubation with 4 mM captopril; gel 3, 5 mM captopril; gel 4, 6 mM captopril; gel 5, 7 mM captopril.
Table 4.
IC50 (mmol/l): concentration of angiotensin converting enzyme (ACE) inhibitor inhibiting 50% of matrix metalloproteinase (MMP) zymographic activity as a measure of the in vitro MMP inhibitory capacity of different ACE inhibitors
| MMP-2 | MMP-9 | |
| Captopril | 2.00 (0.06) | 1.65 (0.18) |
| Lisinopril | 5.40 (0.28) | 7.86 (0.43) |
| Ramipril | 2.10 (0.09) | 2.01 (0.30) |
Values are mean (SD).
DISCUSSION
The extracellular matrix (ECM) is responsible for cardiac cell alignment and myocardial structural integrity. Synthesis and degradation of ECM are balanced and tightly controlled in normal myocardium.11,12 In pathophysiological processes, an imbalance of proteinase/antiproteinase systems occurs, resulting in quantitative and qualitative alterations in matrix composition.13 All four categories of proteinases (serine, cysteine, and aspartic proteinases, and metalloproteinases) have been implicated in the proteolytic process. As collagen represents the major structural protein of ECM, it has long been postulated that collagenolytic MMP plays a pivotal role in cardiac remodelling. Inhibition of the proteolytic activity of MMP has been suggested as a potential therapeutic target in various diseases, including heart failure.13–21
The data we present here show different regulatory patterns of MMP depending on the underlying aetiology of heart failure. MMP-9 upregulation is a common feature of matrix activation in terminal heart failure, irrespective of the underlying disease, whereas in our study MMP-2 was upregulated only in IDCM and remained unchanged in coronary artery disease—in line with a previous report.22
The fact that we could not detect increased MMP mRNA expression in failing myocardium suggests the presence of additional regulatory mechanisms. In particular, the strikingly low mRNA expression of MMP-9 in coronary diseased myocardium compared with normal myocardium contrasts with the large amount of activated MMP-9 measured by ELISA. A possible explanation for this phenomenon is the circadian synthesis of MMP, with inactive proenzymes kept in stock, implying a chronological imbalance between MMP synthesis and activation.23,24
Sources of MMP are fibroblasts, myocytes, endothelial cells, and infiltrating inflammatory cells.12,25 Monocytes stimulated by tumour necrosis factor α, which have been found to be increased in heart failure, are capable of expressing MMP-9.26–28 It is possible that these cells may serve as carriers of MMP-9 synthesised before they infiltrate the myocardium. This hypothesis is supported by the observation that in reperfusion of infarcted myocardium, infiltrating neutrophils are the predominant source of MMP-9 and activating enzymes.25 The source and regulation of MMP activity in normal and diseased myocardium, with a focus on MMP-9 in coronary artery disease, warrants further investigation.
Another interesting question arises: what is the result of the abundant MMP-9 in the myocardium in coronary artery disease and IDCM? Heart failure is a progressive disease. MMP-9 is a key enzyme in matrix component degradation, suggesting a role in matrix remodelling that results in left ventricular enlargement and depression of systolic function. Some of the known substrates of MMP-9 are collagen fragments, gelatin, elastin, aggrecan, versican, and fibronectin. Some of these have important roles in the remodelling process. MMP-9 has been implicated in angiogenesis and apoptosis.16,29,30 Rouet-Benzineb and colleagues localised MMP-9 immunohistochemically in cardiomyocytes and showed myosin heavy chain cleavage by MMP-9 in vitro, suggesting a direct impairment of the contractile apparatus by MMP-9.31 There is increasing evidence in studies from transgenic animals that MMP mediates crucial steps in the development of heart failure. The fact that MMP-9 knockout mice survive with no apparent defect has been related to redundancy—expression of related proteins covering for the same function is assumed.32,33 As the disease progresses, the knockout animals show a decreased ability to adapt or compensate. After experimental myocardial infarction in an MMP-9 knockout model, it was convincingly shown that the targeted deletion of MMP-9 attenuated left ventricular enlargement and collagen accumulation.32 In another mouse myocardial infarction model it was found that MMP-9 deficiency protected against cardiac rupture.34
Except for ACE inhibitors, no other drugs used in the care of heart failure patients have been shown to inhibit MMP activity directly. However, the MMP inhibitory capacity of ACE inhibitors determined by zymographic assay in vitro is not easily transferable to the situation in vivo. There are no data on tissue ACE inhibitor concentrations in human heart, but selective enrichment of ACE inhibitors has been assumed.35
Concentrations of ACE inhibitors in zymography are necessarily higher than in blood owing to the need to permeate the SDS-PAGE gel. Other technical points should be mentioned, such as different solubility, different pH, and the application of active metabolites of prodrugs (such as ramiprilate in place of ramipril). However, a non-specific effect of ACE inhibitors on MMP can be excluded by its reversibility by an excess of zinc.
The finding of a direct inhibitory effect of ACE inhibitors on MMP in vitro is of uncertain clinical relevance. The course of the proteolytic activity in the human disease process—from starter event to end stage heart failure—remains unknown. The question of timing arises; do ACE inhibitors affect remodelling more in the earlier stages or in the end stage of heart failure? Do patients who do not respond to ACE inhibition predominantly develop end stage heart failure requiring transplantation?
Conclusions
No definitive statement on the relevance of the interaction of ACE inhibitors and the MMP system is possible. In considering the assumed influence of ACE inhibitors on MMP, we have emphasised the differences in MMP inhibiting capacity between the various different ACE inhibitors.
Acknowledgments
This work was supported in part by the German government (Bundesministerium für Bildung und Forschung), grant 2,5 VKF, FKZ 01779602 and National Institutes of Health grant R01-HL-71010. We wish to thank Silke Schreiber and Ulla-Brigitte Köhler for their excellent technical assistance.
Abbreviations
ACE, angiotensin converting enzyme
ECM, extracellular matrix
GAPDH, glyceraldehyde-3-phosphodehydrogenase
IDCM, idiopathic dilated cardiomyopathy
MMP, matrix metalloproteinase
RT-PCR, reverse transcriptase polymerase chain reaction assays
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
TIMP, tissue inhibitor of matrix metalloproteinases
REFERENCES
- 1.Pitt B, Poole-Wilson P, Segal R, et al. Effects of losartan versus captopril on mortality in patients with symptomatic heart failure: rationale, design, and baseline characteristics of patients in the losartan heart failure survival study – ELITE II. J Card Fail 1999;5:146–54. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation 1997;96:4065–82. [DOI] [PubMed] [Google Scholar]
- 3.Wollert KC, Studer R, Doerfer K, et al. Differential effects of kinins on cardiomyocyte hypertrophy and interstitial collagen matrix in the surviving myocardium after myocardial infarction in the rat. Circulation 1997;95:1910–17. [DOI] [PubMed] [Google Scholar]
- 4.Sorbi D, Fadly M, Hicks R, et al. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int 1993;44:1266–72. [DOI] [PubMed] [Google Scholar]
- 5.McElmurray JH, Mukherjee R, New RB, et al. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: comparative effects on left ventricular function and geometry. J Pharmacol Exp Ther 1999;291:799–811. [PubMed] [Google Scholar]
- 6.Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation 1996;93:841–2. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi SC, Matsubara L, Weber KT. Direct extraction and estimation of collagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem 1993;26:191–8. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann MH, Kuhnert H, Muller S et al. Monocyte chemoattractant protein 1 (MCP-1) gene expression in dilated cardiomyopathy. Cytokine 1998;10:739–46. [DOI] [PubMed] [Google Scholar]
- 11.Weber KT, Sun Y, Tyagi SC, et al. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol 1994;26:279–92. [DOI] [PubMed] [Google Scholar]
- 12.Tyagi SC, Kumar SG, Haas SJ, et al. Post-transcriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol 1996;28:1415–28. [DOI] [PubMed] [Google Scholar]
- 13.Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991;5:2145–54. [PubMed] [Google Scholar]
- 14.Spinale FG, Coker ML, Krombach SR, et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: effects on left ventricular dimensions and function. Circ Res 1999;85:364–76. [DOI] [PubMed] [Google Scholar]
- 15.Spinale FG, Coker ML, Bond BR, et al. Myocardial matrix degradation and metalloproteinase activation in the failing heart: a potential therapeutic target. Cardiovasc Res 2000;46:225–38. [DOI] [PubMed] [Google Scholar]
- 16.Yip D, Ahmad A, Karapetis CS, et al. Matrix metalloproteinase inhibitors: applications in oncology. Invest New Drugs 1999;17:387–99. [DOI] [PubMed] [Google Scholar]
- 17.Moore G, Liao S, Curci JA, et al. Suppression of experimental abdominal aortic aneurysms by systemic treatment with a hydroxamate-based matrix metalloproteinase inhibitor (RS 132908). J Vasc Surg 1999;29:522–32. [DOI] [PubMed] [Google Scholar]
- 18.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest 2000;105:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez RE, Hartwig W, Antoniu BA, et al. Effect of matrix metalloproteinase inhibition on pancreatic cancer invasion and metastasis: an additive strategy for cancer control. Ann Surg 2000;231:644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escalante T, Franceschi A, Rucavado A, et al. Effectiveness of batimastat, a synthetic inhibitor of matrix metalloproteinases, in neutralizing local tissue damage induced by BaP1, a hemorrhagic metalloproteinase from the venom of the snake bothrops asper. Biochem Pharmacol 2000;60:269–74. [DOI] [PubMed] [Google Scholar]
- 21.Haq M, Shafii A, Zervos EE, et al. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer Res 2000;60:3207–11. [PubMed] [Google Scholar]
- 22.Spinale FG, Coker ML, Heung LJ, et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000;102:1944–9. [DOI] [PubMed] [Google Scholar]
- 23.Grant GM, Cobb JK, Castillo B, et al. Regulation of matrix metalloproteinases following cellular transformation. J Cell Physiol 1996;167:177–83. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JT, Li H, Dillon L, et al. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res 2000;46:307–15. [DOI] [PubMed] [Google Scholar]
- 25.Lindsey M, Wedin K, Brown MD, et al. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation 2001;103:2181–7. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Nakanishi I, Yamashita K, et al. Matrix metalloproteinase-9 (92 kDa gelatinase/type IV collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci 1993;104:991–9. [DOI] [PubMed] [Google Scholar]
- 27.Leber TM, Balkwill FR. Regulation of monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble factor (MMPSF). Br J Cancer 1998;78:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, McCluskey K, Fujii K, et al. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol 1998;161:3071–6. [PubMed] [Google Scholar]
- 29.Michaelides MR, Curtin ML. Recent advances in matrix metalloproteinase inhibitors research. Curr Pharm Des 1999;5:787–819. [PubMed] [Google Scholar]
- 30.Pugin J, Verghese G, Widmer MC, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 1999;27:304–12. [DOI] [PubMed] [Google Scholar]
- 31.Rouet-Benzineb P, Buhler JM, Dreyfus P, et al. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation. Eur J Heart Fail 1999;1:337–52. [DOI] [PubMed] [Google Scholar]
- 32.Ducharme A, Frantz S, Aikawa M, et al. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 2000;106:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson HP. A tenascin knockout with a phenotype. Nat Genet 1997;17:5–7. [DOI] [PubMed] [Google Scholar]
- 34.Heymans S, Luttun A, Nuyens D, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 1999;5:1135–42. [DOI] [PubMed] [Google Scholar]
- 35.Johnston CI, Fabris B, Yamada H, et al. Comparative studies of tissue inhibition by angiotensin converting enzyme inhibitors. J Hypertens Suppl 1989;7:S11–16. [PubMed] [Google Scholar]