Abstract
Objective: To determine whether there is a relation between impairment of lung diffusion and reduced exercise capacity in chronic heart failure.
Design: 40 patients with heart failure in stable clinical condition and 40 controls participated in the study. All subjects underwent standard pulmonary function tests plus measurements of resting lung diffusion (carbon monoxide transfer, Tlco), pulmonary capillary volume (Vc), and membrane resistance (Dm), and maximal cardiopulmonary exercise testing. In 20 patients and controls, the following investigations were also done: (1) resting and constant work rate Tlco; (2) maximal cardiopulmonary exercise testing with inspiratory O2 fractions of 0.21 and 0.16; and (3) rest and peak exercise blood gases. The other subjects underwent Tlco, Dm, and Vc measurements during constant work rate exercise.
Results: In normoxia, exercise induced reductions of haemoglobin O2 saturation never occurred. With hypoxia, peak exercise uptake (peak V̇o2) decreased from (mean (SD)) 1285 (395) to 1081 (396) ml/min (p < 0.01) in patients, and from 1861 (563) to 1771 (457) ml/min (p < 0.05) in controls. Resting Tlco correlated with peak V̇o2 in heart failure (normoxia < hypoxia). In heart failure patients and normal subjects, Tlco and peak V̇o2 correlated with O2 arterial content at rest and during peak exercise in both normoxia and hypoxia. Tlco, Vc, and Dm increased during exercise. The increase in Tlco was greater in patients who had a smaller reduction of exercise capacity with hypoxia. Alveolar–arterial O2 gradient at peak correlated with exercise capacity in heart failure during normoxia and, to a greater extent, during hypoxia.
Conclusions: Lung diffusion impairment is related to exercise capacity in heart failure.
Keywords: heart failure, exercise, lung function
Several lines of evidence suggest that gas diffusion across the alveolar–capillary membrane influences exercise capacity in patients with heart failure: first, lung transfer capacity for carbon monoxide (Tlco) correlates with exercise capacity1–3; second, improvement in clinical symptoms and exercise capacity with angiotensin converting enzyme (ACE) inhibitors is correlated with an increase in Tlco4,5; third, oxygen uptake kinetics can be altered by manipulation of lung diffusion6; and fourth, exercise performance can be improved in patients with heart failure by increasing the partial pressure of oxygen physiologically—that is, by exercising patients below sea level7—or artificially, by increasing the inspired oxygen fraction8 or by positive pressure ventilation.9 However, the physiological relation between reduction in exercise capacity and Tlco impairment in heart failure is uncertain. This is because in heart failure, neither an exercise induced reduction in arterial oxygen content (Cao2), nor the more easily measured exercise induced desaturation of haemoglobin—which is considered the clinical hallmark of Tlco impairment—is commonly observed.10 Nonetheless the role of Cao2 in regulating the amount of oxygen available for the muscles, the so called oxygen delivery, during exercise is pivotal in heart failure. Indeed, oxygen delivery depends on Cao2 and cardiac output, but in heart failure the increase in cardiac output during exercise is blunted. Several physiological factors regulate Cao2 including the following: the systemic arterial Po2 (Pao2), which depends on alveolar Po2, the gas exchange surface area, and the alveolar–arterial oxygen pressure gradient (ΔP[A−ao2]); the haemoglobin concentration; and the shape of the haemoglobin dissociation curve for oxygen. All these factors undergo exercise induced changes.11 Gas exchange surface and ΔP[A−ao2] are reflected by Tlco.12
To evaluate the relation between lung diffusion impairment and exercise intolerance in chronic heart failure we studied in the correlations between Tlco, Cao2, and exercise capacity during normoxic and hypoxic conditions both at rest and during exercise. Different levels of inspired oxygen fraction (Fio2) were chosen because during hypoxia, both Pao2 and haemoglobin saturation with oxygen (Sao2) are in the linear portion of the S shaped haemoglobin dissociation curve for oxygen, making it easier to study and understand the role of Tlco in regulating Cao2 and exercise capacity.
METHODS
Patient population
Forty patients with stable heart failure (mean (SD) age 61.9 (6.4) years; 30 male, 10 female) and 40 healthy controls (57.6 (9.6) years; 28 male, 12 female) participated in the study.
All the heart failure patients were in New York Heart Association (NYHA) functional class II or III and belonged to a cohort of heart failure patients regularly followed in our heart failure clinic. Heart failure aetiology was: ischaemic cardiomyopathy (15), idiopathic (11), alcoholic (7), HIV related (4), and related to antitumour drugs (3). Exclusion criteria included: a left ventricular ejection fraction > 35% by echocardiography, the presence of periodic breathing during exercise, primary pulmonary disease, unstable angina, recent myocardial infarction, and artificial pacemakers. Ten patients were active smokers, 20 were previous smokers (defined as patients who quit smoking more than five years ago), and 10 had never smoked. Treatment was stable and included: digitalis (13), diuretics (34), ACE inhibitors (29), angiotensin I blockers (8), β blockers (18), and amiodarone (18).
Healthy controls were chosen from patients’ relatives and hospital employees or their friends. Eighteen were smokers, eight were previous smokers, and 14 never smoked. None was involved in regular exercise programmes.
The study was approved by the local ethics committee and all subjects provided their written informed consent.
Pulmonary function evaluation
Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured in triplicate and calculated according to the American Thoracic Society criteria,13 using a mass flow sensor (2200 Sensor Medics, Yorba Linda, California, USA). Maximum voluntary ventilation (MVV) was assumed to be either MVV measured in 12 seconds or FEV1 × 40, whichever was highest.14 Predicted values are from Quanjer and colleagues, and Jones.15,16 Tlco was measured with the single breath constant expiratory flow technique. Tlco data are reported as absolute values or as a percentage of predicted.17 Molecular diffusion of carbon monoxide across the alveolar–capillary membrane (Dm) and pulmonary capillary blood volume (Vc) were measured according to the method of Roughton and Forster.18 Tlco, Dm, and Vc are linked by the following equation:
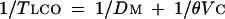 |
where θ is the rate of reaction of carbon monoxide with haemoglobin and is inversely proportional to Po2 in the alveolar air (PAo2). Therefore subjects inspired a gas mixture with 0.3% CH4, 0.3% CO, and 0.3% C2H2 balanced with nitrogen with three different O2 fractions equal to 20%, 40%, and 60%, respectively. This procedure allows measurement of Tlco at different Pao2 values, thereby causing θ to vary and enabling calculation of Dm and Vc graphically.18
Cardiopulmonary exercise testing
Maximal cardiopulmonary exercise tests (Vmax 29C, Sensor Medics) were done on a cycle ergometer (Ergometrics-800, Sensor Medics), using a personalised ramp protocol aimed at achieving peak exercise in around 10 minutes.19 Data are reported as absolute values or as a percentage of the V̇o2 max predicted.14 Ventilation and partial pressures of inspiratory and expiratory gases were measured on a breath by breath basis. The anaerobic threshold was calculated by “V slope” analysis (plot of V̇co2 divided by V̇o2) V̇ V̇o2/heart rate.
In subjects in group A (see below), a small brachial artery catheter was inserted to allow easy and repeatable arterial blood sampling. In these subjects two cardiopulmonary exercise tests were performed while they breathed a gas mixture with an Fio2 of 21% or 16% (equivalent to the oxygen tension found at an altitude of around 2000 m) in random order. Arterial blood was sampled in triplicate at rest, after at least 10 minutes of quiet breathing on the cycle ergometer, and again at peak exercise. These samples were used to measure haemoglobin concentrations, oxygen and carbon dioxide tensions, Cao2, and pH (ABL 520, Radiometer, Copenhagen, Denmark). PAo2 was determined from the equation:
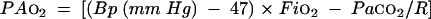 |
where Bp = barometric pressure, Fio2 = oxygen inspired fraction, Paco2 = arterial carbon dioxide partial pressure, and R = respiratory gas exchange ratio.
Study design
The first part of the protocol was the same for all subjects. Both patients and control subjects underwent standard pulmonary function tests, resting Tlco, Dm, and Vc measurements (in the sitting position) and an incremental cardiopulmonary exercise test. At least one cardiopulmonary exercise test was performed before the formal study to familiarise the subjects with the techniques and procedures. Subjects were then randomly allocated into two groups, A and B, each comprising 20 heart failure patients and 20 normal controls.
Investigations in group A
In group A, Tlco was also measured with the subjects sitting on the ergometer and after three and five minutes of light exercise (20% of the maximum workload achieved). The cardiopulmonary exercise tests, with an Fio2 of 21% and 16%, were done on the following days. The order of the two tests was randomised and a resting interval of more than six hours was allowed between each test.
Investigations in group B
In group B, only one cardiopulmonary exercise test was performed to assess exercise capacity while the subjects were breathing room air. Tlco, Dm, and Vc were measured with the subjects sitting on the cycle ergometer and during light exercise (20% of maximum workload achieved) which lasted around five minutes.
Statistical analysis
Data are presented as mean (SD). Correlations were obtained by linear regression analysis and the best fit method. Differences were evaluated by analysis of variance (ANOVA) and the unpaired t test, applying the Bonferroni correction for multiple comparisons as appropriate. Multivariate stepwise regression model (SPSS 9.0) was used to identify independent predictors of Tlco and peak exercise oxygen consumption (peak V̇o2). All variables with a univariate probability value of p < 0.05 were included.
RESULTS
Pulmonary function and exercise capacity (all subjects)
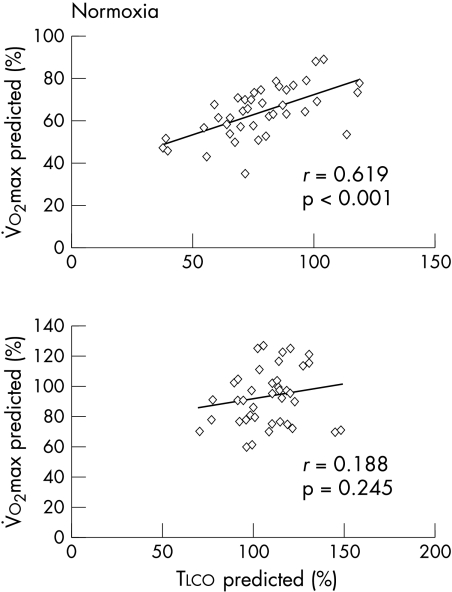
Results of the pulmonary function tests were consistent with a mild restrictive defect in the heart failure patients (table 1). Compared with the normal controls, resting Tlco was reduced in the heart failure group owing to a reduction in Dm with a normal Vc (table 1). V̇o2 at peak exercise and at anaerobic threshold was 1285 (376)/800 (140) and 1866 (540)/1010 (290) ml/min in patients and controls, respectively (p < 0.01 for both conditions). Oxygen pulse at peak exercise was 10.0 (2.7) and 12.5 (3.5) ml/beat in patients and normal subjects, respectively (p < 0.05). In heart failure patients (fig 1, upper panel) but not in normal subjects (lower panel) resting Tlco was significantly correlated with normoxic peak V̇o2. To avoid confounding by variables such as age, sex, or anthropometric measurements, both resting Tlco and peak V̇o2 are reported as per cent of predicted normal values.
Table 1.
Standard pulmonary function and lung diffusion tests in the whole study population
| Heart failure patients (n=40) | Normal controls (n=40) | |
| FEV1 (% pred) | 86 (20)* | 114 (21) |
| FVC (% pred) | 74 (12)* | 106 (12) |
| FEV1 / FVC | 115 (15) | 108 (10) |
| MVV (% pred) | 85 (20) | 118 (17) |
| Tlco (ml/min/mm Hg) | 19.9 (5.5)* | 28.0 (6.1) |
| Tlco (% pred) | 77 (19)* | 108 (17) |
| Dm (ml/min/mm Hg) | 29.0 (10.6)* | 45.4 (12.8) |
| Vc (ml) | 103.8 (40.8) | 103.3 (38.2) |
Data are presented as mean (SD).
*p < 0.01 v normal controls.
Dm, molecular diffusion for carbon monoxide across the alveolar capillary membrane; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MVV, maximum voluntary ventilation; pred, Tlco, lung transfer capacity for carbon monoxide; Vc, capillary blood volume.
Figure 1.
Peak V̇o2 during normoxia v resting lung transfer capacity for carbon monoxide (Tlco) (reported as per cent of predicted) in heart failure patients (upper panel, n = 40) and healthy controls (lower panel, n = 40).
Exercise capacity in hypoxic condition (group A)
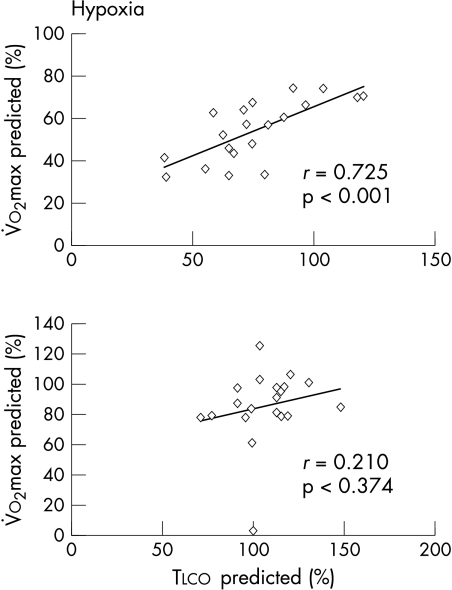
With normoxia, peak exercise V̇o2 was 1285 (395) and 1861 (563) ml/min in patients and controls, respectively. The maximum work rate achieved was 101 (36) W in the patients and 163 (54) W in the controls. With hypoxia (Fio2 = 16%), peak V̇o2 reduced to 1081 (396) in patients and to 1771 (457) and in normal subjects, respectively (p < 0.01 and p < 0.05 v normoxic condition); the maximum work rate was reduced to 87 (34) W in patients and to 157 (52) W in normal subjects (p < 0.01 and p < 0.05 v normoxic condition). With hypoxia, in both patients and normal subjects ventilation was increased at rest and throughout the test compared with normoxic levels, but not at peak exercise (table 2). Resting Tlco was correlated with peak V̇o2 obtained under hypoxic conditions (fig 2), with an R value greater than in normoxic conditions (0.725 and 0.619, respectively). Resting Tlco, Dm, and Vc did not predict the reduction in exercise capacity with hypoxia, either in patients or in controls.
Table 2.
Ventilation, tidal volume, and respiratory rate at rest and on peak exercise under normoxic and hypoxic conditions in patients with heart failure (n = 20) and normal controls (n = 20) (group A)
| Normoxia | Hypoxia | |||
| Rest | Peak exercise | Rest | Peak exercise | |
| Heart failure patients | ||||
| Ventilation (l/min) | 11 (2) | 58 (18)* | 28 (6)† | 57 (19)* |
| Tidal volume (l) | 0.6 (0.1) | 1.6 (0.4)* | 1.3 (0.2)*† | 1.6 (0.4)* |
| Respiratory rate (beats/min) | 19 (4)* | 37 (7) | 21 (4)* | 36 (7) |
| Normal controls | ||||
| Ventilation (l/min) | 10 (2) | 69 (19) | 28 (5)† | 72 (18) |
| Tidal volume (l) | 0.6 (0.1) | 2.1 (0.6) | 1.5 (0.3)† | 2.3 (0.6) |
| Respiratory rate (beats/min) | 15 (4) | 34 (5) | 18 (3) | 32 (8) |
Data are presented as mean (SD).
*p < 0.01 v normal controls; †p < 0.01 v normoxia.
Figure 2.
Peak V̇o2 during hypoxia v resting lung transfer capacity for carbon monoxide (Tlco) (both reported as per cent of predicted) in heart failure patients (upper panel, n = 20) and healthy subjects (lower panel, n = 20) (group A). In chronic heart failure, the correlation between Tlco and peak V̇o2 increased further during hypoxia.
Tlco during exercise
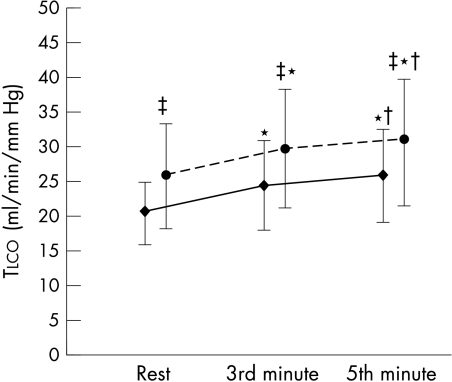
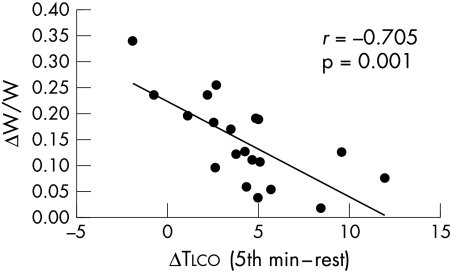
Tlco increased during exercise at low work rates (20% of maximum workload) in both normal subjects and heart failure patients (fig 3, data from group A). This increase reflected the increase in Vc and Dm (table 3, data from group B). The patients with the greater capacity to increase Tlco during submaximal exercise were those who showed a smaller reduction in exercise capacity with hypoxia (fig 4, data from group A).
Figure 3.
Lung transfer capacity for carbon monoxide (Tlco) at rest (on the bicycle ergometer) and at the third and fifth minute of constant workload exercise (20% of peak exercise workload). Circles, normal subjects; diamonds, chronic heart failure patients. *p < 0.01 v rest; †p < 0.01 v 3rd minute value; ‡p < 0.01 v chronic heart failure patients.
Table 3.
CO transfer (Tlco) subcomponents during submaximal exercise (group A, 20 heart failure patients and 20 normal controls)
| Heart failure subjects | Normal controls | |||
| Rest | 5th minute | Rest | 5th minute | |
| Dm (ml/min/mm Hg) | 29.1 (8.4)* | 36.4 (12.8)*† | 46.9 (14.0) | 51.3 (19.2)† |
| Dm/Va | 5.4 (1.3)* | 6.6 (2.5)*† | 8.0 (2.1) | 8.7 (2.5)† |
| Vc (ml) | 109 (42) | 145 (65)*† | 104 (39) | 134 (31)† |
Data are presented as mean (SD).
*p < 0.01 v normal controls; †p < 0.01 v values at rest.
Dm, molecular diffusion for carbon monoxide across the alveolar–capillary membrane; Dm/Va, Dm normalised for alveolar volume; Vc, pulmonary capillary blood volume.
Figure 4.
Reduction of exercise capacity with hypoxia. ΔW/W = [maximum workload achieved in normoxia − maximum workload achieved in hypoxia]/maximum workload achieved in normoxia. ΔTlco = differences in lung diffusing capacity for carbon monoxide between the fifth minute of exercise and rest in heart failure patients. Patients with the greatest capability to increase Tlco during exercise are those who show the smallest reduction in exercise capacity in hypoxia.
Blood gas values and exercise capacity in normoxic and hypoxic conditions
Haemoglobin concentration, Po2, Sao2, Cao2, alveolar po2, and ΔP[A−ao2] at rest and peak exercise in normoxic and hypoxic conditions are reported in table 4; each datum is the mean of three measurements. In the normoxic condition Po2, Cao2, alveolar Po2, and ΔP[A−ao2] increased during exercise in both patients and normal controls. With hypoxia the resting data were comparable between the normal subjects and the patients. At peak exercise, Po2 and Sao2 decreased compared with resting values in both patients and normal controls.
Table 4.
Haemoglobin concentration, Po2, Sao2, Cao2, and Paco2 at rest and during peak exercise under normoxic and hypoxic conditions (group A, 20 heart failure patients and 20 normal controls)
| Normoxia | Hypoxia | |||
| Rest | Peak exercise | Rest | Peak exercise | |
| Heart failure patients | ||||
| Hb (g/dl) | 14.0 (1.5) | 14.9 (1.6)* | 14.3 (1.4) | 15.0 (1.4)* |
| Po2 (mm Hg) | 86 (6) | 99 (11)* | 68 (9)† | 61 (8)*† |
| Sao2 (%) | 97.2 (0.8) | 97.7 (1.2) | 94.9 (2.0)† | 92.1 (3.1)*† |
| Cao2 (ml/100 ml) | 18.3 (2.0) | 19.5 (2.2)* | 18.1 (1.9) | 18.5 (2.3)*† |
| pH | 7.42 (0.03) | 7.40 (0.04)* | 7.46 (0.05) | 7.44 (0.04)* |
| PAo2 (mm Hg) | 100 (8) | 119 (5)* | 79 (4)† | 84 (4)*† |
| ΔP[A−ao2] (mm Hg) | 14.7 (7.6) | 20.5 (10.0)* | 11.1 (8.8)† | 23.0 (7.8)* |
| Paco2 (mm Hg) | 36.8 (4.5) | 33.3 (5.5)* | 31.2 (4.1)† | 32.1 (4.4) |
| Normal controls | ||||
| Hb (g/dl) | 14.1 (1.4) | 15.1 (1.4)* | 14.2 (1.5) | 15.2 (1.3)* |
| Po2 (mm Hg) | 92.9 (6.0) | 98.5 (7.0)* | 74.6 (9.5)† | 67.6 (7.2)*† |
| Sao2 (%) | 97.8 (0.6) | 97.8 (0.5) | 96.2 (1.6)† | 93.8 (2.2)*† |
| Cao2 (ml/100 ml) | 18.5 (1.9) | 19.8 (1.8)* | 18.3 (1.8) | 19.1 (1.7)*† |
| pH | 7.42 (0.02) | 7.37 (0.04)* | 7.46 (0.04)† | 7.39 (0.04)*† |
| PAo2 (mm Hg) | 97.0 (4.8) | 117.2 (4.5)* | 77.4 (6.8)† | 88.1 (5.4)*† |
| ΔP[A−ao2] (mm Hg) | 4.2 (6.5) | 18.6 (5.6)* | 3.0 (5.3) | 20.4 (4.9)* |
| Paco2 (mm Hg) | 36.9 (3.3) | 33.9 (3.8)* | 30.6 (4.2)† | 30.5 (4.0)† |
Values are presented as mean (SD).
*p < 0.05 v rest; †p < 0.05 v normoxia.
Cao2, arterial oxygen content; ΔP[A−ao2], alveolar–arterial pressure difference for oxygen; Hb, haemoglobin; Paco2, arterial carbon dioxide tension; PAo2, alveolar oxygen pressure; Po2, arterial oxygen tension; Sao2 , haemoglobin saturation with oxygen.
In group A patients there was a weak but significant correlation between resting Tlco and (1) Sao2, (2) Cao2, and (3) arterial Po2 at rest and at peak exercise, in both normoxic and hypoxic conditions; resting Tlco was also weakly correlated with haemoglobin at peak exercise during both normoxia and hypoxia (table 5). In contrast to the patients, in normal subjects resting Tlco was significantly correlated only with haemoglobin and Cao2, measured during normoxia and hypoxia both at rest and at peak exercise. None of the heart failure patients showed significant (> 3%) haemoglobin desaturation either at rest or peak exercise in normoxic conditions (mean Sao2 at rest 97.2 (0.8)%, range 94.7–98.4%; mean Sao2 at peak exercise 97.5 (1.4)%, range 93.2–99.0%). Correlations between peak V̇o2 and Sao2, haemoglobin, Cao2, and Po2 in both patients and normal controls are reported in table 6. The correlations between peak V̇o2 and Cao2 and haemoglobin were significant in patients and controls in all the conditions studied.
Table 5.
Correlations between resting carbon monoxide transfer (Tlco) and haemoglobin oxygen saturation, arterial oxygen content, haemoglobin, and arterial oxygen tension (group A, 20 heart failure patients and 20 normal controls)
| Sao2 | Cao2 | Hb | Po2 | |
| Heart failure patients | ||||
| Normoxia, rest | R=0.633, p<0.01 | R=0.438, p=0.06 | R=0.394, NS | R=0.674, p<0.01 |
| Normoxia, peak exercise | R=0.503, p<0.02 | R=0.499, p<0.03 | R=0.483, p<0.05 | R=0.488, p<0.02 |
| Hypoxia, rest | R=0.479, p<0.03 | R=0.503, p<0.03 | R=0.425, NS | R=0.417, p=0.06 |
| Hypoxia, peak exercise | R=0.541, p<0.01 | R=0.500, p<0.03 | R=0.476, p<0.03 | R=0.541, p<0.01 |
| Normal controls | ||||
| Normoxia, rest | R=−0.140, NS | R=0.645, p<0.01 | R=0.655, p<0.01 | R=−0.148, NS |
| Normoxia, peak exercise | R=−0.216, NS | R=0.688, p<0.01 | R=0.690, p<0.01 | R=0.164, NS |
| Hypoxia, rest | R=−0.210, NS | R=0.678, p<0.01 | R=0.684, p<0.01 | R=−0.192, NS |
| Hypoxia, peak exercise | R=−0.087, NS | R=0.563, p<0.01 | R=0.603, p<0.01 | R=−0.148, NS |
Data were obtained from means of three samples.
Cao2, arterial oxygen content; Hb, haemoglobin; Po2, arterial oxygen tension; Sao2, haemoglobin saturation with oxygen.
Table 6.
Correlations of peak oxygen consumption with haemoglobin saturation with oxygen, arterial oxygen content, haemoglobin, and arterial oxygen tension (group A, 20 heart failure patients and 20 normal controls)
| Sao2 | Cao2 | Hb | Po2 | |
| Heart failure patients | ||||
| Normoxia, rest | R=0.515, p<0.02 | R=0.509, p<0.02 | R=0.474, p<0.05 | R=0.569; p<0.01 |
| Normoxia, peak exercise | R=0.333, NS | R=0.557, p<0.01 | R=0.561, p<0.01 | R=0.340, NS |
| Hypoxia, rest | R=0.243, NS | R=0.478, p<0.04 | R=0.462, p=0.05 | R=0.187, NS |
| Hypoxia, peak exercise | R=0.462, p<0.04 | R=0.542, p<0.01 | R=0.538, p<0.02 | R=0.525, p<0.02 |
| Normal controls | ||||
| Normoxia, rest | R=0.315, NS | R=0.597, p<0.01 | R=0.615, p=0.01 | R=−0.003, NS |
| Normoxia, peak exercise | R=0.143, NS | R=0.674, p<0.01 | R=0.696, p<0.01 | R=−0.434, NS |
| Hypoxia, rest | R=−0.306, NS | R=0.539, p<0.02 | R=0.662, p<0.02 | R=−0.315, NS |
| Hypoxia, peak exercise | R=−0.291, NS | R=0.532, p<0.02 | R=0.677, p<0.01 | R=−0.231, NS |
Data were obtained from means of three samples.
Cao2, arterial oxygen content; Hb, haemoglobin; Po2, arterial oxygen tension; Sao2, haemoglobin saturation with oxygen.
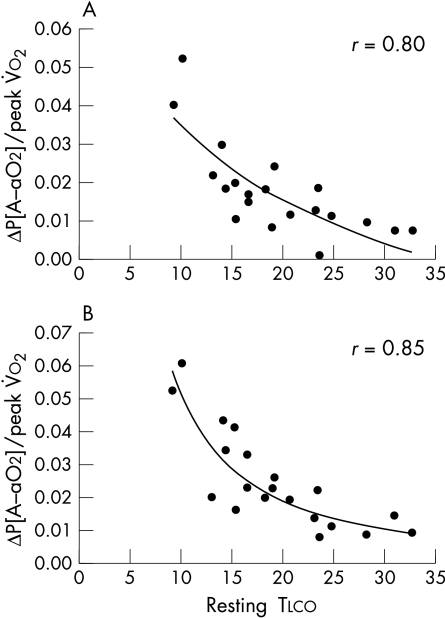
The ΔP[A−ao2] value in the patients was greater at rest with normoxia than in the normal controls (table 4). However, the increase at peak exercise was greater in the controls than in the patients (14.4 (6.4) v 5.8 (10.0) mm Hg, p < 0.01). With hypoxia the increase in the ΔP[A−ao2] value from rest to peak was 11.9 (5.9) mm Hg and 15.9 (9.1) mm Hg in patients and in normal subjects, respectively (NS). There was a significant correlation between resting Tlco and ΔP[A−ao2] at peak exercise in normoxia and at rest and peak exercise in hypoxia in patients but not in normal subjects (table 7). Adjusting ΔP[A−ao2] for peak V̇o2 strengthened this correlation at peak exercise in the patients, and made it evident in the normal subjects (table 7). Best fit analysis showed that a curvilinear relation significantly improved the correlation of ΔP[A−ao2], adjusted for peak V̇o2, with Tlco in both normoxic (fig 5, upper panel) and hypoxic conditions (fig 5, lower panel).
Table 7.
Correlation between resting carbon monoxide transfer (Tlco) and alveolar–arterial pressure difference for oxygen (group A, 20 heart failure patients and 20 normal controls)
| Heart failure patients | Normal controls | |
| ΔP[A−ao2], normoxia, rest | R=−0.439, p=0.06 | R=0.06, NS |
| ΔP[A−ao2]), normoxia, peak exercise | R=−0.516, p<0.02 | R=−0.211, NS |
| ΔP[A−ao2], hypoxia, rest | R=−0.502, p<0.02 | R=−0.106, NS |
| ΔP[A−ao2], hypoxia, peak exercise | R=−0.625, p<0.01 | R=−0.207, NS |
| ΔP[A−ao2]/V̇o2, normoxia, rest | R=−0.384, NS | R=−0.022, NS |
| ΔP[A−ao2]/V̇o2, normoxia, peak exercise | R=−0.720, p<0.01 | R=−0.488, NS |
| ΔP[A−ao2]/V̇o2, hypoxia, rest | R=−0.431, p=0.06 | R=0.049, NS |
| ΔP[A−ao2]/V̇o2, hypoxia, peak exercise | R=−0.794, p<0.01 | R=−0.791, p<0.01 |
ΔP[A−ao2], alveolar–arterial pressure difference for oxygen; V̇o2, oxygen uptake.
Figure 5.
Correlation between alveolar–arterial O2 differences at peak exercise divided by peak V̇o2 (ΔP[A−ao2]/peak V̇o2) v resting diffusing capacity for carbon monoxide (Tlco) in normoxia (upper panel) and hypoxia (lower panel).
DISCUSSION
This study contains several observations aimed at elucidating the complex interplay between lung diffusion abnormalities and impairment of exercise capacity in patients with heart failure.
In the first place, our study confirms that in patients with heart failure, resting Tlco correlates with peak V̇o2. Although we were only able to show a correlation and not a cause–effect link, we believe that a causal relation between impaired Tlco and reduced exercise capacity exists; indeed when the physiological impact of Tlco reduction is increased, as with hypoxia, the correlation between Tlco and peak V̇o2 is high.
Second, our study provides evidence that in heart failure patients, even though resting and peak exercise Sao2, Po2, and Cao2 are in the normal range, their values correlate with Tlco, that Tlco increases during exercise as a result of increases in both Vc and Dm, and that patients who have the greatest capability to increase their Tlco during exercise are those who have the smallest reduction in exercise capacity with hypoxia.
Finally, in both normoxic and hypoxic conditions, the value of the ΔP[A−ao2] differences is related to Tlco, so when oxygen flow across the alveolar capillary membrane has to increase, as with exercise, or is impaired, as in hypoxia, the ΔP[A–ao2] difference increases more the lower the resting Tlco value.
The patients we studied belong to a cohort of subjects regularly followed in our heart failure clinic. They were in stable clinical condition and, as in several previous reports,2–5,20,21 results of standard pulmonary function tests and Tlco showed mild restrictive lung disease and impairment of diffusion. As previously reported,2,3 Tlco impairment at rest correlates with exercise capacity. However, even if several pieces of evidence suggest a link between Tlco and exercise capacity, the physiological meaning of this correlation remains controversial because, in contrast with patients with pulmonary disease, exercise induced haemoglobin desaturation is rare in patients with heart failure. A cardiopulmonary exercise test with a reduced O2 fraction is a safe test used to assess exercise capacity at moderate altitude.22 With hypoxia, peak V̇o2 and maximum work rate were reduced in both heart failure patients and normal controls. It is noteworthy that the correlation between Tlco and exercise capacity was high in the hypoxic tests (fig 2), a condition where Tlco impairment is likely to became more important.
In normal subjects we found a correlation between Tlco and Cao2, probably mediated by haemoglobin. In heart failure patients, in contrast, resting Tlco correlated with arterial Sao2, Po2, and Cao2 both at rest and during peak exercise in normoxic conditions; this suggests that in patients who are not able to increase their cardiac output adequately during exercise, the Cao2 at rest and its increase during exercise11,23 became relevant determinants of oxygen delivery (and therefore of exercise capacity) (table 3). Accordingly, even if the Sao2, arterial Po2, and Cao2 values which we measured in the normoxic condition are within the so called “normal range” (table 4),24 values at the lower end of this range are functionally relevant. In other words, it makes a difference for a heart failure patient whether they have a haemoglobin of 12 mg/dl or 15 mg/dl, or an Sao2 of 94% or 98%. Furthermore, our data confirm the role of an increase in haemoglobin as a determinant of exercise capacity.11
To our knowledge, blood gas analyses during exercise in hypoxia have not previously been reported in patients with heart failure. In normal subjects as well as in heart failure patients, resting Tlco was significantly correlated with haemoglobin and Cao2, measured at peak exercise, suggesting that in hypoxia Cao2 becomes a relevant determinant of exercise capacity. The observed reduction of Po2 and Sao2 is counterbalanced by an increase in haemoglobin concentration,25 which serves to obviate an undesirable reduction of Cao2 during exercise. Two explanations for the decrease in arterial Po2 at peak exercise with hypoxia are likely. In the first place, there could be a hypoxia induced increase in pulmonary shunting because of hypoxic pulmonary vasoconstriction enhancing the ventilation–perfusion mismatch; secondly, the pulmonary capillary transit time could be too short for a reduced alveolar Po2 to achieve an equilibrium between alveolar and capillary Po2 pressures. Indeed with hypoxia the A–ao2 gradient increased compared with normoxia, both at rest and during peak exercise (table 6). An inadequate exercise induced increase in ventilation during hypoxia is unlikely because Paco2 levels did not increase.
Smith and colleagues3 recently showed that Tlco increases during light exercise in heart failure patients.3 Our findings are consistent with that report and provide new information about the cause of the exercise induced increase in Tlco. Tlco depends on membrane diffusion capacity and capillary volume, and both were increased during exercise in our heart failure patients and normal controls. The increase in Vc is likely to be caused by pulmonary vessel recruitment. The exercise induced increase in Dm is more difficult to understand. The increase in Dm during exercise confirms that Dm is not a fixed value but can increase. This observation is in line with the suggestion that Tlco should be used as an antifailure treatment target.3,4 It is not possible to measure Tlco or its components reliably at peak exercise when haemoconcentration can further increase Tlco by increasing the surface of the alveoli in contact with the red blood cells.25 We measured Tlco during light exercise (around 20% of the maximum workload achieved) and therefore we cannot say whether this value represents the maximum possible increase in Tlco or not. We used a light workload to show that Tlco can be increased and that at the same increment of work rate the increase in Tlco correlates with the capacity of the subjects to adjust to exercise under hypoxic conditions. Indeed we showed that patients who increase Tlco most during exercise are those with the least reduction in hypoxia induced exercise capacity—meaning that the increase in Tlco during exercise can be viewed as a compensatory mechanism.
Conclusions
While none of the present evidence, when considered in isolation, proves a causal role of Tlco impairment in the reduced exercise capacity of patients with heart failure, collectively the following findings strongly suggest that its role is indeed causal:
the resting Tlco correlates with exercise capacity and that this correlation is increased with hypoxia
a low but “normal” arterial haemoglobin content, Sao2, and Cao2 are associated with reduced exercise performance in heart failure patients
a reduced capacity to increase Tlco during submaximal effort correlates with the reduction of exercise capacity with hypoxia
if resting Tlco is low at peak exercise with hypoxia, then the ΔP[A−ao2] difference shows the greatest oxygen gradient.
Abbreviations
Cao2, arterial oxygen content
Dm
membrane resistance
ΔP[A−ao2], alveolar
arterial oxygen pressure gradient; FEV1, forced expiratory volume in one second
Fio2, inspired oxygen fraction
FVC, forced vital capacity
MVV, maximum voluntary ventilation
Pao2, systemic arterial oxygen tension
Sao2, haemoglobin saturation with oxygen
Tlco, carbon monoxide transfer
Vc, pulmonary capillary blood volume
V̇o2
oxygen uptake
REFERENCES
- 1.Sue DY, Oren A, Hansen JE, et al. Diffusing capacity for carbon monoxide as a predictor of gas exchange during exercise. N Engl J Med 1987;316:1301–6. [DOI] [PubMed] [Google Scholar]
- 2.Puri S, Baker BL, Dutka DP, et al. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure. Circulation 1995;91:2769–74. [DOI] [PubMed] [Google Scholar]
- 3.Smith AA, Cowburn PJ, Parker ME, et al. Impaired pulmonary diffusion during exercise in patients with chronic heart failure. Circulation 1999;100:1406–10. [DOI] [PubMed] [Google Scholar]
- 4.Guazzi M, Marenzi GC, Alimento M, et al. Improvement of alveolar capillary diffusing capacity in chronic heart failure and counteracting effects of aspirin. Circulation 1997;95:1930–6. [DOI] [PubMed] [Google Scholar]
- 5.Guazzi M, Marenzi GC, Melzi G, et al. Angiotensin-converting enzyme inhibition facilitates alveolar-capillary gas transfer and improves ventilation perfusion coupling in patients with left ventricular dysfunction. Clin Pharmacol Ther 1999;65:319–27. [DOI] [PubMed] [Google Scholar]
- 6.Koike A, Wasserman K, McKenzie DK, et al. Evidence that diffusion limitation determines oxygen uptake kinetics during exercise in humans. J Clin Invest 1990;86:11698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abinader EG, Sharif DS, Goldhammer E. Effects of low altitude on exercise performance in patients with congestive heart failure after healing of acute myocardial infarction. Am J Cardiol 1999;83:383–7. [DOI] [PubMed] [Google Scholar]
- 8.Moore P, Weston A, Hughes JMB, et al. Effects of increased inspired oxygen concentrations on exercise performance in chronic heart failure. Lancet 1992;339:850–3. [DOI] [PubMed] [Google Scholar]
- 9.O’ Donnel DE, D’Arsigny C, Ray S, et al. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med 1999;160:1804–11. [DOI] [PubMed] [Google Scholar]
- 10.Wasserman K, Zhang YY, Gitt A, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997;96:2221–7. [DOI] [PubMed] [Google Scholar]
- 11.Perego G, Marenzi GC, Guazzi M, et al. Contribution of pO2, p50 and hemoglobin concentration to changes in arterio-venous oxygen content during exercise in patients with heart failure. J Appl Physiol 1996;80:623–31. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M. Alveolar capillary membrane dysfunction in chronic heart failure: pathophysiology and therapeutic implications. Clin Sci 2000;98:633–41. [PubMed] [Google Scholar]
- 13.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–18. [DOI] [PubMed] [Google Scholar]
- 14.Hansen J, Sue D, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129:S49–55. [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Tammeling GJ, Cotes JE, et al. Standardized lung function testing. Eur Respir J 1993;6:1–99. [Google Scholar]
- 16.Jones NL. Clinical exercise testing, 3rd ed. Philadelphia: WB Saunders, 1988:306–11.
- 17.Huang YC, Helms MJ, MacIntyre NR. Normal values for single exhalation diffusing capacity and pulmonary capillary blood flow in sitting, supine position, and during mild exercise. Chest 1994;105:501–6. [DOI] [PubMed] [Google Scholar]
- 18.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol 1957;11:290–302. [DOI] [PubMed] [Google Scholar]
- 19.Hansen JE, Casaburi R, Cooper DM, et al. Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur J Appl Physiol 1988;57:140–5. [DOI] [PubMed] [Google Scholar]
- 20.Hosenpud JD, Stibolt TA, Atwal K, et al. Abnormal pulmonary function specifically related to congestive heart failure: comparison of patients before and after cardiac transplantation. Am J Med 1990;88:493–6. [DOI] [PubMed] [Google Scholar]
- 21.Wright RS, Levine MS, Bellamy PE, et al. Ventilatory and diffusion abnormalities in potential heart transplant recipients. Chest 1990;98:816–20. [DOI] [PubMed] [Google Scholar]
- 22.Agostoni P, Cattadori G, Guazzi M, et al. Effects of simulated altitude-induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med 2000;109:450–5. [DOI] [PubMed] [Google Scholar]
- 23.Agostoni PG, Wasserman K, Perego GB, et al. Oxygen transport to muscle during exercise in chronic congestive heart failure secondary to idiopathic cardiomyopathy. Am J Cardiol 1997;79:1120–4. [DOI] [PubMed] [Google Scholar]
- 24.Lentner C. Geigy scientific tables, vol 3. Basel: Ciba-Geigy Ltd, 1984:71–7.
- 25.Agostoni PG, Wasserman K, Guazzi M, et al. Exercise induced hemoconcentration in heart failure due to dilated cardiomyopathy. Am J Cardiol 1999;83:278–80. [DOI] [PubMed] [Google Scholar]