Abstract
Objective: To evaluate the relation between changes in ejection fraction during the first three months after acute myocardial infarction and myocardial viability.
Patients: Myocardial viability was assessed using low dose dobutamine echocardiography in 107 patients at mean (SD) 3 (1) days after acute myocardial infarction. Cross sectional echocardiography was repeated three months later. Left ventricular volumes and ejection fraction were determined from apical views using the Simpson biplane formula.
Results: In patients with viability, ejection fraction increased by 4.4 (4.3)%; in patients without viability it remained unchanged (0.04 (3.6)%; p < 0.001). A ≥ 5% increase in ejection fraction was present in 21 of 107 patients (20%). Receiver operating characteristic analysis showed that myocardial viability in ≥ 2 segments predicted this increase in ejection fraction with a sensitivity of 81% and a specificity of 65%. Multivariate logistic regression analysis was used to define which clinical and echocardiographic variables were related to ≥ 5% improvement in ejection fraction. Myocardial viability, non-Q wave infarction, and anterior infarction all emerged as independent predictors, myocardial viability being the best (χ2 = 14.5; p = 0.0001). Using the regression equation, the probability of ≥ 5% improvement in ejection fraction for patients with a non-Q wave anterior infarct with viability was 73%, and for patients with a Q wave inferior infarct without viability, only 2%.
Conclusions: Myocardial viability after acute myocardial infarction is the single best predictor of improvement in ejection fraction. In combination with infarct location and Q wave presence, the probability of ≥ 5% improvement can be estimated in individual patients at the bedside.
Keywords: echocardiography, myocardial infarction, myocardial viability
The left ventricular ejection fraction is one of the most powerful predictors of mortality after acute myocardial infarction.1–3 Changes in the ejection fraction occurring after the acute phase have been assessed in several studies.4–9 These showed that, although there was often no overall change, there was a great deal of interpatient variation—indeed, in several reports a significant improvement in the ejection fraction was found, especially in patients with sustained successful reperfusion and in those with the lowest ejection fraction at baseline.9–14
Improvements in the ejection fraction may have a beneficial effect on survival, and this is likely to be greatest in patients with severe left ventricular dysfunction.2,7 It has been found that the final value achieved and the degree of improvement are more important predictors of mortality than the value of the ejection fraction at the time of the acute event.15,16 Thus it is important to determine which variables identify patients who will show an improvement in the ejection fraction. The presence of myocardial viability is likely to be one of those variables. However, previous studies using low dose dobutamine echocardiogaphy in patients with acute myocardial infarction have concentrated exclusively on predicting reversible dysfunction on a regional basis.17–22 Our aim in this study was therefore to determine the clinical and echocardiographic predictors of improvement in left ventricular ejection fraction using low dose dobutamine echocardiography in an unselected group of patients with acute myocardial infarction.
METHODS
Patients
The study population consisted of 107 patients with acute myocardial infarction who had been enrolled in a prospective postinfarction low dose dobutamine echocardiographic study. Patients treated with and without thrombolysis or primary coronary angioplasty, with first or recurrent infarction, and those with Q wave or non-Q wave infarction were eligible.
The following categories of patients were not eligible or were excluded from the analyses: no baseline wall motion abnormalities (11); technically difficult echocardiographic studies (13); postinfarction angina (6); reinfarction (5); infarction complicated by severe haemodynamic instability (3); sustained ventricular tachycardia or ventricular fibrillation occurring > 24 hours after admission (6); additional revascularisation procedures before inclusion (1); mitral valve replacement before the second echocardiogram (1); technically inadequate echocardiogram for quantitative analysis (14); and absence of echocardiographic follow up (16).
All patients gave informed consent for their participation in this prospective study, which was approved by the science and ethics committee of our institutions.
Echocardiography
Patients underwent low dose dobutamine echocardiography at a mean (SD) of 3 (1) days (range 2–5 days) after the onset of myocardial infarction. β Blockers were withdrawn 24 hours before the test. After baseline cross sectional echocardiography, dobutamine was given intravenously at doses of 5 and 10 μg/kg/min, for five minutes at each dose. Standard parasternal and apical views were recorded at baseline and at the end of the dobutamine infusion on VHS videotape for subsequent off-line analysis. The echocardiographic images were digitised and displayed side by side in quadscreen format to facilitate the comparison of images. Follow up cross sectional echocardiography was repeated 3 (1) months later.
Data analysis
Echocardiograms were reviewed by two experienced observers (FN, PMJV). A third observer (OK) was consulted in cases of disagreement. Wall motion was evaluated using a 13 segment model of the left ventricle and a four point scoring system: 1, normokinesia; 2, hypokinesia; 3, akinesia; 4, dyskinesia.23 Attention was paid to both endocardial motion and systolic wall thickening. The assessment was based on the digitised images displayed in quadscreen format and on a review of the images recorded on videotape. Myocardial viability was defined as an improvement in thickening during dobutamine infusion in two or more contiguous infarct zone segments. Improved thickening was defined as a change from akinesia or dyskinesia to hypokinesia or normal wall thickening, and from hypokinesia to normal wall thickening. A change from dyskinesia to akinesia was not considered to be improved wall thickening. The wall motion score index was calculated by summing the scores for each segment and dividing by the number of segments analysed.
Left ventricular end diastolic and end systolic volumes were determined from apical two and four chamber views using the Simpson biplane formula according to the recommendations of the American Society of Echocardiograpy.24 The ejection fraction was calculated as [end diastolic minus end systolic volume]/end diastolic volume × 100%. Intraobserver and interobserver variabilities in the echocardiographic assessment of the ejection fraction in our laboratory were 3.4% and 4.1%, respectively. Patients were considered to have improvement in global left ventricular function if the ejection fraction increased by ≥ 5% from baseline to follow up.
Statistical analysis
Continuous data are presented as mean (SD). Group differences in continuous data were assessed using the unpaired Student’s t test. Differences between proportions were assessed by χ2 analysis; a Fisher exact test was used when appropriate. Changes in left ventricular function over time were analysed by paired Student’s t test. Variables that were significantly different by unpaired t testing or by χ2 testing between patients with and without improvement in the ejection fraction were submitted to univariate regression analysis.
Clinical variables selected for analysis were age, sex, previous myocardial infarction, infarct location, enzymatic infarct size, thrombolytic treatment, primary coronary angioplasty, reperfusion treatment, diabetes, a history of hypertension, and drug treatment prescribed at hospital discharge.
Echocardiographic variables tested were wall motion score index at rest, left ventricular end diastolic and end systolic volume index, ejection fraction, and the presence of myocardial viability.
Variables that showed a significant correlation with improvement in the ejection fraction were included in the multivariate stepwise logistic regression model to determine the independent correlates. A probability value of p < 0.05 was considered significant. The regression equation was used to obtain the estimated probability of improvement in ejection fraction for any combination of the three independent variables. To select the optimum number of viable segments for predicting improvement in ejection fraction, receiver operating characteristic (ROC) curves were used.
RESULTS
Patients
In the study group as a whole, the left ventricular ejection fraction did not change significantly (from 49 (8)% at baseline to 51 (9)% at follow up); there was, however, considerable interpatient variability, ranging from an increase of 16.4% to a decrease of 7.7%. On the basis of the presence or absence of improvement in left ventricular ejection fraction, the patients were divided into two groups: 21 showed a ≥ 5% increase and were assigned to the improvement group, while 86 showed no improvement.
Table 1 summarises baseline characteristics of the two groups. There were no significant differences in age, sex, frequency of coronary risk factors, mode of treatment, or enzymatic infarct size. Ejection fraction at baseline, left ventricular volume indices, and wall motion score indices did not differ between the two groups. Patients in the improvement group more often had anterior myocardial infarction (76% v 42%, p = 0.005) and were less likely to have Q wave infarction (43% v 78%, p = 0.002) than patients without improvement. Myocardial viability was more likely to be present in patients with improvement (81% v 35%, p < 0.0001).
Table 1.
Baseline characteristics
| Variable | Improvement group (n=21) | No improvement group (n=86) | p Value |
| Age (years) | 56 (13) | 60 (11) | NS |
| Male | 18 (86%) | 74 (86%) | NS |
| Anterior infarct | 16 (79%) | 36 (42%) | 0.005 |
| Inferior infarct | 5 (24%) | 50 (58%) | 0.005 |
| Previous infarction | 4 (19%) | 11 (13%) | NS |
| Thrombolytic treatment | 15 (71%) | 57 (66%) | NS |
| Primary PTCA | 4 (19%) | 7 (8%) | NS |
| Reperfusion treatment | 19 (90%) | 64 (74%) | NS |
| Q wave infarction | 9 (43%) | 67 (78%) | 0.002 |
| Peak creatine kinase (U/l) | 607 (1332) | 1603 (1290) | NS |
| Peak MB fraction (U/l) | 140 (128) | 150 (94) | NS |
| Drugs at discharge | |||
| β Blockers | 18 (86%) | 67 (78%) | NS |
| ACE inhibitors | 8 (38%) | 40 (47%) | NS |
| Nitrates | 8 (38%) | 33 (38%) | NS |
| Calcium antagonists | 3 (14%) | 15 (17%) | NS |
| End diastolic volume index (ml/m2) | 63 (8) | 63 (14) | NS |
| End systolic volume index (ml/m2) | 33 (8) | 33 (13) | NS |
| Ejection fraction (%) | 48 (7) | 49 (8) | NS |
| Wall motion score index | 1.61 (0.24) | 1.56 (0.27) | NS |
| Number of viable segments | 2.4 (1.16) | 0.90 (1.08) | <0.001 |
| Viability | 17 (81%) | 30 (35%) | <0.001 |
Values are mean (SD) or n (%).
ACE, angiotensin converting enzyme; PTCA, percutaneous transluminal coronary angioplasty.
At follow up, the wall motion score index and the end systolic volume index were lower in patients with improvement (1.35 (0.25) v 1.51 (0.31), p < 0.05; and 28 (7) v 35 (16) ml/m2, p < 0.05, respectively). Although the left ventricular end diastolic volume index did not differ between the groups, left ventricular dilatation—defined as an increase of more than 10% in the end diastolic volume index at follow up—was found less often in patients with improvement (0% v 27%, p = 0.007).
Before the three month follow up examination, revascularisation procedures were performed in five of the 21 patients with improvement (24%) compared with 18 of the 86 patients without improvement (21%) (NS). During this period, 19 patients suffered from recurrent infarction or unstable angina; six of these were in the improvement group and 13 in the no improvement group (NS). During the mean follow up period of 20 (7) months, three patients died of cardiac causes. All of these were in the no improvement group (NS).
Predictors of improvement in ejection fraction
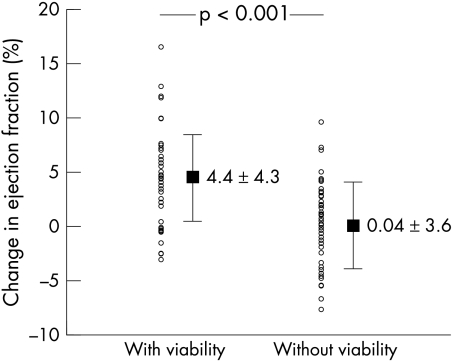
At univariate analysis, anterior infarct location, non-Q wave myocardial infarction, and the presence of myocardial viability were all found to be predictive of improvement in the ejection fraction; of these, myocardial viability was the best predictor (χ2 = 14.5, p = 0.0001). In patients with viability, the ejection fraction increased by 4.4 (4.3)%, while it remained unchanged in patients without viability (0.04 (3.6)%, p < 0.001) (fig 1).
Figure 1.
Change in left ventricular ejection fraction between baseline and three months after myocardial infarction in patients with and without viability.
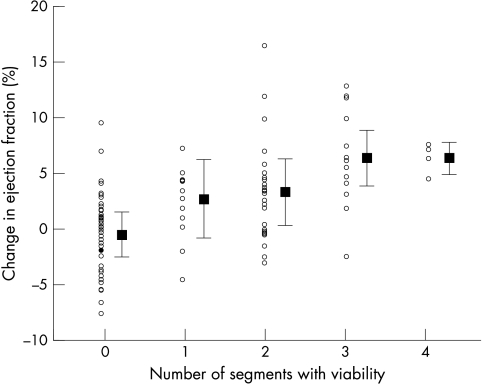
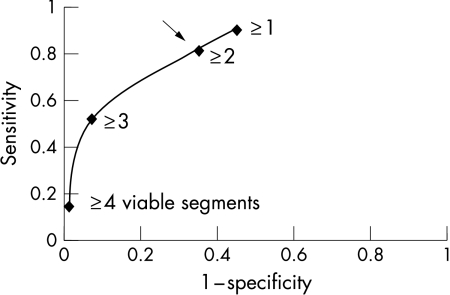
Figure 2 shows the relation between the number of segments with viability and the change in the ejection fraction at three months. On the basis of ROC analysis, the optimum operating point on the curve that best predicted an increase of ≥ 5% in the ejection fraction was ≥ 2 segments of viable myocardium (fig 3). This operating point was associated with a sensitivity of 81% and a specificity of 65%.
Figure 2.
Relation between the number of segments with viability detected by low dose dobutamine echocardiography and the change in the ejection fraction from baseline to three months in patients with acute myocardial infarction.
Figure 3.
Empirical receiver operating characteristic curve for prediction of improvement in ejection fraction for the number of segments with viability.
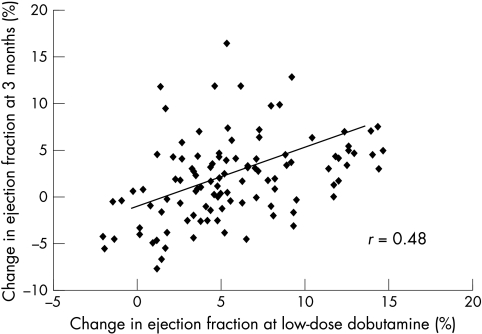
The change in the ejection fraction during low dose dobutamine echocardiography was predictive of improvement (χ2 = 8.6, p = 0.0034). In patients with improvement, the change in the ejection fraction on low dose dobutamine echocardiography was 8.0 (4.2)%, compared with 5.1 (3.8)% in the no improvement group (p = 0.003). Figure 4 shows the relation between the change in the ejection fraction on low dose dobutamine echocardiography and the change in the ejection fraction at the three months follow up (r = 0.48, p < 0.001).
Figure 4.
Scatterplot showing correlation of changes in ejection fraction during low dose dobutamine with those observed three months after acute myocardial infarction.
When multivariate logistic regression analysis was undertaken, myocardial viability, non-Q wave myocardial infarction, and anterior infarct location all emerged as independent predictors of improvement in the ejection fraction. Using the regression equation, the probability of improvement in the ejection fraction for patients with a non-Q wave anterior myocardial infarct with viability was 73%. Patients with a Q wave inferior myocardial infarct without viability had only a 2% probability of improvement. The estimated probabilities of improvement in the ejection fraction for any combination of the three independent variables are shown in table 2.
Table 2.
Probability of ≥ 5% improvement in the ejection fraction
| Viability | No viability | |
| Anterior infarct, non-Q wave | 0.73 | 0.30 |
| Anterior infarct, Q wave | 0.32 | 0.07 |
| Inferior infarct, non-Q wave | 0.42 | 0.10 |
| Inferior infarct, Q wave | 0.11 | 0.02 |
DISCUSSION
Determinants of improvement in ejection fraction: previous studies
Several studies have assessed the sequential changes in global left ventricular function during the acute, subacute, and chronic phases after myocardial infarction.4–14 Large thrombolytic trials, using contrast ventriculography or radionuclide angiography to assess ejection fraction, have shown no overall change in global ejection fraction during the first weeks after thrombolytic treatment.4,5,9 Improvement in regional infarct zone function is often counteracted by a decrease in non-infarct zone hyperkinesia.4,8 However, despite the absence of large group changes, there is considerable individual improvement.
One of the most important determinants of improvement in the ejection fraction appears to be sustained successful reperfusion.9–12,25,26 Harrison and colleagues carried out early and one week catheterisations in 542 patients treated with intravenous thrombolysis for acute myocardial infarction9: acute TIMI (thrombolysis in myocardial infarction trial) grade 3 flow in the infarct related artery was independently associated with improvement in the left ventricular ejection fraction at one week. Meijer and colleagues showed an increase in the ejection fraction from 51 (10)% within 48 hours after thrombolysis to 55 (11)% after three months in 95 patients with sustained reperfusion, compared with no change in 34 patients with reocclusion of the infarct related artery.27
In addition to sustained successful reperfusion, the extent of left ventricular dysfunction in the acute phase is independently associated with improvement in the ejection fraction.9,11,12,14 Marzoll and colleagues studied 137 patients with acute myocardial infarction and found that those with the lowest baseline ejection fraction within 24 hours of acute infarction were the most likely to improve before hospital discharge.11 This may have been related in part to the statistical phenomenon of regression to the mean. Other factors related to improvement in global ejection fraction are anterior infarct location7,12,27 and non-Q wave infarction,14 in agreement with our study. None of the abovementioned factors, however, would enable a clinician at the bedside to identify those patients with a high likelihood of improvement in global left ventricular function.
Prognostic significance of serial changes in ejection fraction
The left ventricular ejection fraction is one of the most powerful predictors of mortality after acute myocardial infarction.1–3 The relation between the ejection fraction and mortality has been described as a hyperbolic curve, with an upturn in mortality occurring at values of less than 40%.2,7 Previous clinical studies have shown that serial measurements of ejection fraction provide additional prognostic information.15,16 Brodie and colleagues followed up 576 hospital survivors of acute myocardial infarction treated with primary angioplasty for 5.3 years.16 Serial measurements of ejection fraction (acute and at six months) were available in 65% of the patients. Multivariate analysis showed that the final achieved ejection fraction and improvement in ejection fraction were more important predictors of mortality than the acute ejection fraction. In patients with heart failure it has also been demonstrated that changes in the ejection fraction have additional prognostic value.28 In the V-HeFT (vasodilator heart failure) trials, a change in ejection fraction of > 5% was the strongest predictor of mortality, even after adjustment for treatment and for baseline ejection fraction.28 Thus it is important to have a readily available diagnostic protocol that enables a clinician at the bedside to identify patients with a high likelihood of improvement in ejection fraction.
Low dose dobutamine echocardiography
Low dose dobutamine echocardiography is one of the currently available techniques for determining the presence of viable myocardium. In several studies the sensitivity and specificity for predicting regional functional improvement after acute myocardial infarction have ranged from 66–86% and from 68–94%, respectively.17–22 All those studies have concentrated exclusively on predicting reversible dysfunction on a regional basis. Prediction of the reversibility of global left ventricular function has only been investigated in patients with left ventricular dysfunction caused by chronic coronary artery disease.29–32 Cornel and colleagues found that dobutamine echocardiography has a sensitivity of 89% and a specificity of 81% in predicting a ≥ 5% improvement in left ventricular ejection fraction after revascularisation.29 In that study, patients with ≥ 4 viable segments (in a 16 segment model and a five point scoring system) are likely to have improved global left ventricular function later. In our study, viability in ≥ 2 segments on low dose dobutamine echocardiography (using a 13 segment model and a four point scoring system) predicted an improvement in the ejection fraction of ≥ 5% with a sensitivity of 81% and a specificity of 65%.
By combining presence or absence of myocardial viability with two simple independent clinical variables (infarct location and Q wave infarction) the probability of improvement could be estimated at the bedside. As mentioned above, patients with a non-Q wave anterior infarct with myocardial viability have a probability of improvement of 73%, whereas patients with a Q wave inferior infarct without myocardial viability have a negligible probability of improvement of 2%.
Study limitations
One of the limitations of our study was the uncontrolled performance of revascularisation procedures before the second echocardiography. However, there was no difference in the number of revascularisation procedures in patients with and without improvement in ejection fraction (24% v 21%), and when the analysis was restricted to the 84 patients without revascularisation, there was no change in the results—myocardial viability, non-Q wave infarction, and anterior infarction remained the independent predictors of improvement in ejection fraction. Furthermore, although medical treatment could not be standardised, there were no differences between patients with and without improvement.
Another limitation of the study was that coronary angiography was not performed routinely. Only 58 of the 107 patients (54%) underwent this procedure at the discretion of the cardiologist. In line with previous studies, there was a trend towards more occluded infarct related arteries in patients without improvement (27% v 9%, NS).9–12,25,26 There was also a trend towards more reperfusion therapy in patients with improvement (90% v 74%) (table 1), but again the small number of patients limits the detection of differences because of insufficient statistical power.
Finally, the number of patients with cardiac death was too small to assess the additional prognostic value of final achieved ejection fraction or improvement in ejection fraction in our study.
Conclusions
The presence of myocardial viability after acute myocardial infarction is the single best predictor of improvement in ejection fraction. Combining this with infarct location and Q wave infarction, the probability of an increase of ≥ 5% in ejection fraction can be estimated at the bedside.
Acknowledgments
We gratefully acknowledge Hans Bosch, Laboratory for Clinical and Experimental Image Processing, Leiden University Hospital, for technical assistance with data analysis. We appreciate the great support provided by our technicians Irma Bekkering, Sylvia Bruinzeel, Anja Folkers, and Beatrix Willemsen.
REFERENCES
- 1.The Multicenter Postinfarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med 1983;309:331–6. [DOI] [PubMed] [Google Scholar]
- 2.Volpi A, De Vita C, Franzosi MG, et al and the ad hoc Working Group of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-2 data base. Determinants of 6-month mortality in survivors of myocardial infarction after thrombolysis. Results of the GISSI-2 data base. Circulation 1993;88:416–29. [DOI] [PubMed] [Google Scholar]
- 3.Stadius ML, Davis K, Maynard C, et al. Risk stratification for 1 year survival based on characteristics identified in the early hours of acute myocardial infarction. The Western Washington Intracoronary Streptokinase Trial. Circulation 1986;74:703–11. [DOI] [PubMed] [Google Scholar]
- 4.Sheehan FH, Braunwald E, Canner P, et al. The effect of intravenous thrombolytic therapy on left ventricular function: a report on tissue-type plasminogen activator and streptokinase from the thrombolysis in myocardial infarction (TIMI phase I) trial. Circulation 1987:75:817–29. [DOI] [PubMed] [Google Scholar]
- 5.The TIMI Study Group. Comparison of invasive and conservative strategies after treatment with intravenous tissue plasminogen activator in acute myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) phase II trial. N Engl J Med 1989;320:618–27. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill W, Timmis GC, Bourdillon PD, et al. A prospective randomized clinical trial of intracoronary streptokinase versus coronary angioplasty for acute myocardial infarction. N Engl J Med 1986;314:812–18. [DOI] [PubMed] [Google Scholar]
- 7.Serruys PW, Simoons ML, Suryapranata H, et al. Preservation of global and regional left ventricular function after early thrombolysis in acute myocardial infarction. J Am Coll Cardiol 1986;7:729–42. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt WG, Sheehan FH, von Essen R, et al. Evolution of left ventricular function after intracoronary thrombolysis for acute myocardial infarction. Am J Cardiol 1989;63:497–502. [DOI] [PubMed] [Google Scholar]
- 9.Harrison JK, Califf RM, Woodlief LH, et al and the TAMI Study Group. Systolic left ventricular function after reperfusion therapy for acute myocardial infarction. An analysis of determinants of improvement. Circulation 1993;87:1531–41. [DOI] [PubMed] [Google Scholar]
- 10.Henzlova MJ, Bourge RC, Papapietro SE, et al. Long-term effect of thrombolytic therapy on left ventricular ejection fraction after acute myocardial infarction. Am J Cardiol 1991;67:1354–9. [DOI] [PubMed] [Google Scholar]
- 11.Marzoll U, Kleiman NS, Dunn JK, et al. Factors determining improvement in left ventricular function after reperfusion therapy for acute myocardial infarction: primacy of baseline ejection fraction. J Am Coll Cardiol 1991;17:613–20. [DOI] [PubMed] [Google Scholar]
- 12.Brodie BR, Weintraub RA, Hansen CJ, et al. Factors that predict improvement in left ventricular ejection fraction after coronary angioplasty for acute myocardial infarction. Cathet Cardiovasc Diagn 1987;13:372–80. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Eisenberg MJ, Schiller NB. Serial assessment of left ventricular function after myocardial infarction. Am Heart J 1995;130:999–1002. [DOI] [PubMed] [Google Scholar]
- 14.Humbert VH, Jabi H, Burger AJ, et al. Late variation in ventricular function after myocardial infarction. Chest 1991;100:28–33. [DOI] [PubMed] [Google Scholar]
- 15.Sheehan FH, Doerr R, Schmidt WG, et al. Early recovery of left ventricular function after thrombolytic therapy for acute myocardial infarction: an important determinant of survival. J Am Coll Cardiol 1988;12:289–300. [DOI] [PubMed] [Google Scholar]
- 16.Brodie BR, Stuckey TD, Kissling G, et al. Importance of infarct-related artery patency for recovery of left ventricular function and late survival after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 1996;28:319–25. [DOI] [PubMed] [Google Scholar]
- 17.Pierard LA, DeLandsheere CM, Berthe C, et al. Identification of viable myocardium by echocardiography during dobutamine infusion in patients with myocardial infarction after thrombolytic therapy: comparison with positron emission tomography. J Am Coll Cardiol 1990;15:1021–31. [DOI] [PubMed] [Google Scholar]
- 18.Barilla F, Gheorghiade M, Alam M, et al. Low-dose dobutamine in patients with acute myocardial infarction identifies viable but not contractile myocardium and predicts the magnitude of improvement in wall motion abnormalities in response to coronary revascularization. Am Heart J 1991;122:1522–31. [DOI] [PubMed] [Google Scholar]
- 19.Smart SC, Sawada S, Ryan T, et al. Low-dose dobutamine echocardiography detects reversible dysfunction after thrombolytic therapy of acute myocardial infarction. Circulation 1993;88:405–15. [DOI] [PubMed] [Google Scholar]
- 20.Previtali M, Poli A, Lanzarini L, et al. Dobutamine stress echocardiography for assessment of myocardial viability and ischemia in acute myocardial infarction treated with thrombolysis. Am J Cardiol 1993;72:124–30G. [DOI] [PubMed] [Google Scholar]
- 21.Salustri A, Elhendy A, Garyfallydis P, et al. Prediction of recovery of ventricular dysfunction after first acute myocardial infarction using low-dose dobutamine echocardiography. Am J Cardiol 1994;74:853–66. [DOI] [PubMed] [Google Scholar]
- 22.Watada H, Ito H, Oh H, et al. Dobutamine stress echocardiography predicts reversible dysfunction and quantitates the extent of irreversibly damaged myocardium after reperfusion of anterior myocardial infarction. J Am Coll Cardiol 1994;24:624–30. [DOI] [PubMed] [Google Scholar]
- 23.Kan G, Visser CA, Koolen JJ, et al. Short and long term predictive value of admission wall motion score in acute myocardial infarction: a cross sectional echocardiographic study of 345 patients. Br Heart J 1986;56:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller N, Shah PM, Crawford M, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Subcommittee on Standards. J Am Soc Echocardiogr 1989;2:358–68. [DOI] [PubMed] [Google Scholar]
- 25.Miketic S, Carsson J, Tebbe U. Improvement of global and regional left ventricular function by percutaneous transluminal coronary angioplasty after myocardial infarction. J Am Coll Cardiol 1995;25:843–7. [DOI] [PubMed] [Google Scholar]
- 26.Kereiakes DJ, Califf RM, George BS, et al, and the TAMI Group. Coronary bypass surgery improves global and regional left ventricular function following thrombolytic therapy for acute myocardial infarction. Am Heart J 1991;112:390–9. [DOI] [PubMed] [Google Scholar]
- 27.Meijer A, Verheugt FWA, v Eenige MJ, et al. Left ventricular function at 3 months after successful thrombolysis. Impact of reocclusion without infarction on ejection fraction, regional function and remodeling. Circulation 1994;90:1706–14. [DOI] [PubMed] [Google Scholar]
- 28.Cintron G, Johnson G Francis G, et al, for the V-HeFT VA Cooperative Studies Group. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. Circulation 1993;87(suppl VI):VI-17–23. [PubMed] [Google Scholar]
- 29.Cornel JH, Bax JJ, Elhendy A, et al. Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease. Implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol 1998;31:1002–10. [DOI] [PubMed] [Google Scholar]
- 30.Vanoverschelde JLJ, D’Hondt AM, Marwick T, et al. Head to head comparison of exercise-redistribution-reinjection thallium single-photon emission computed tomography and low-dose dobutamine echocardiography for prediction of reversibility of chronic left ventricular ischemic dysfunction. J Am Coll Cardiol 1996;28:432–42. [DOI] [PubMed] [Google Scholar]
- 31.Meluzin J, Cigarroa CG, Brickner E, et al. Dobutamine echocardiography in predicting improvement in global left ventricular systolic function after coronary bypass or angioplasty in patients with healed myocardial infarcts. Am J Cardiol 1995;76:877–80. [DOI] [PubMed] [Google Scholar]
- 32.Bax JJ, Cornel JH, Visser FC, et al. Prediction of recovery of myocardial dysfunction after revascularization. Comparison of FDG/thallium SPECT, thallium stress-reinjection SPECT and dobutamine echocardiography. J Am Coll Cardiol 1996;28:558–64. [DOI] [PubMed] [Google Scholar]