Abstract
Objective: To investigate the possible causes of abnormal blood pressure control in light chain related (primary, AL) amyloidosis.
Design: Cardiovascular, autonomic, and respiratory response to passive tilting were investigated in 51 patients with primary amyloidosis (mean (SEM) age 56 (2) years) and in 20 age matched controls. Spontaneous fluctuations in RR interval, respiration, end tidal carbon dioxide, blood pressure, and skin microcirculation were recorded during supine rest and with tilting. The values were subjected to spectral analysis to assess baroreflex sensitivity and the autonomic modulation of cardiac and vascular responses.
Setting: Tertiary referral centre.
Results: Autonomic modulation of the heart and blood pressure was nearly absent in the patients with amyloidosis: thus baroreflex sensitivity and the low frequency (0.1 Hz) fluctuations in all cardiovascular signals were severely reduced (p < 0.01 or more), as were respiratory fluctuations in the RR interval, and no change was observed upon tilting. Despite reduced autonomic modulation, blood pressure remained relatively stable in the amyloid group from supine to tilting. End tidal carbon dioxide was reduced in the amyloid patients (p < 0.001) indicating persistent hyperventilation; the breathing rate correlated inversely with the fall in blood pressure on tilting (p < 0.05).
Conclusions: In primary amyloidosis, pronounced abnormalities in arterial baroreflexes and cardiovascular autonomic modulation to the heart and the vessels may be partly compensated for by hyperventilation at a slow breathing rate.
Keywords: amyloidosis, postural hypotension, heart rate variability
Primary amyloidosis is a rare systemic disease caused by deposition of monoclonal light chains as fibrillar tissue deposits in different organs, including the heart, peripheral blood vessels, and peripheral and autonomic nerves. As a result, autonomic neuropathy is common and has been reported in almost one quarter of all patients with primary amyloidosis,1,2 though most of the available data refer to the familial type.3,4
In patients with either primary2 or familial amyloidosis,5 abnormalities in heart rate variability are observed more commonly than postural hypotension. Thus, although depressed heart rate variability is an early marker of autonomic dysfunction, the most important point to be assessed is the autonomic control of the vessels. It is possible that compensating factors may delay the clinical onset of autonomic failure, which becomes manifest with the appearance of symptomatic postural hypotension. Possible disturbances in the neural control of the blood vessels and the function of the arterial baroreflex have not so far been studied, despite the frequent occurrence of postural hypotension in amyloidosis and the poor prognosis associated with its occurrence.1,4 The autonomic modulation of blood vessel function can be evaluated non-invasively by combined power spectral analysis of heart rate, blood pressure, and microcirculatory fluctuations,6,7 while the same methodology allows measurement of the arterial baroreflex.8,9 Finally, recent studies have underlined the importance of extravascular factors (such as respiration and physical manoeuvres) as possible compensatory factors in orthostatic hypotension.10,11
To assess the role of impaired autonomic control of the heart and blood vessels in the postural hypotension of amyloidosis, we evaluated autonomic cardiovascular modulation and cardiac vagal baroreflex sensitivity in response to passive tilting in a group of patients and healthy controls. In addition we tested whether an alteration in the spontaneous respiratory dynamics might be implicated in the mechanisms leading to or protecting against postural hypotension.
METHODS
Subjects
The protocol was approved by our local ethics committee, and informed consent was obtained from all participants.
We studied 51 consecutive patients (mean (SD) age 56 (2) years) with a histological diagnosis of amyloidosis and evidence of clonal plasma cell dyscrasia. The time from diagnosis was 19 (3) months (range 1–102 months). Amyloidosis was confirmed by biopsy in every patient. We also studied 20 age matched controls (mean age 54 (1) years).
Patients with reactive, familial, senile, or localised amyloidosis were excluded. The presence of a clonal plasma cell dyscrasia was confirmed by the detection of a monoclonal immunoglobulin in the serum or urine of every patient by high resolution immunofixation using anti-isotype-specific rabbit antisera (Dako, Glostrup, Denmark).12 The concentration of monoclonal protein was determined by densitometry.
Clinical data on the subjects at the time of the study are summarised in table 1. Standard bidimensional echocardiographic evaluation was used to provide estimates of interventricular septal thickness and fractional shortening. Serum concentrations of total proteins and creatinine, packed cell volume, and 24 hour proteinuria were assessed by standard laboratory methods.
Table 1.
Main clinical features of 51 patients with primary amyloidosis
| Feature | Frequency (%) |
| Male/female ratio: 1.59 | |
| Number of organs involved | |
| 0 | 4.3 |
| 1 | 54.3 |
| 2 | 24.0 |
| 3 | 17.4 |
| Predominant organ involved | |
| Kidney | 52.2 |
| Heart | 17.4 |
| Liver | 15.2 |
| Skin | 4.3 |
| Lungs | 2.2 |
| Peripheral nervous system | 4.3 |
| None | 4.3 |
| Total serum protein <60 g/l | 41 |
| Presence of serum monoclonal component | 91 |
| Serum monoclonal component >10 g/l | 14 |
| IgG/IgA/IgM/LC/biclonal | 35/10/10/37.5/7.5 |
| κ/λ | 27/73 |
| Presence of urine monoclonal component | 82 |
| IgG/IgA/LC/biclonal | 19.5/8.3/61.1/11.1 |
| κ/λ | 26/74 |
Disease progression was defined after at least three months of follow up from the time of the initial assessments by fulfilment of at least one of the following criteria:
worsening of the function of organs involved: kidney—doubling of urinary protein excretion if this was initially below 5 g/day, or an increase of more than 50% if it was above 5 g/day; reduction of creatinine clearance by more than 50%; heart—increase of more than 2 mm in the interventricular septal thickness, or a decrease of more than 20% in the ejection fraction; liver—increase in size of more than 2 cm below the costal margin, or at least a 50% increase in serum alkaline phosphatase; neuropathy—electromyographic worsening
any evidence of an increase in size of the amyloid deposits.
Progression was classified as 1 if at least one of these criteria was present, or 0 if none was present.
Protocol
The temperature and humidity in the room were maintained at 20°C (±1) and 60% (±10), respectively, by air conditioning. After 20–30 minutes of supine rest and familiarisation with the laboratory, recordings were obtained during spontaneous breathing (four minutes) and during controlled breathing at 15 breaths/min (four minutes) in the supine position. The same recordings were also obtained during 60° tilting (Akron tilt table, Ipswich, Suffolk, UK). Breathing frequency was controlled at 15 breaths/min to avoid spurious slow breaths interfering with the spontaneous, non-respiration mediated, low frequency (LF) oscillations of the cardiovascular signals.
Spontaneous respiration data were used to evaluate the respiratory variables. To achieve the greatest spontaneity of breathing pattern during these recordings, patients and controls were not told that the recordings had begun. Thus the controlled breathing protocol allowed us to obtain spectral estimations of cardiovascular signals without the possible interference of slow respiration in the LF band, while the spontaneous breathing protocol allowed us to obtain information about normal ventilatory patterns and to determine whether or not the subjects were hyperventilating.
In each condition, we recorded the ECG from the chest leads and blood pressure by a continuous non-invasive applanation technique at the radial artery (Pilot model, Colin Tonometry, San Antonio, Texas, USA).13 Autonomic modulation of the skin microcirculation was assessed in the left index finger by photoplethysmography,9,14 using a previously validated method: briefly, finger photoplethysmography—like laser Doppler flowmetry—evaluates the backscattering of infrared light from a small area of the skin (estimated in the range of 1 mm3); previous studies have shown that the fluctuations observed depend on rhythmic changes in both volume and flow at the level of the skin arterioles. Neither technique is strictly quantitative, as they only measure the amount of backscattered light with respect to a source of fixed intensity. Interindividual comparisons can be made only if the intensity of the light source and the gain of the signal are constant in all subjects; as a result, the signal has to be expressed in arbitrary terms. In this study we defined the “arbitrary unit” as the mV output of the signal, having allowed the infrared source to absorb 10 mW of power.7,9,14,15
The respiratory signal was continuously monitored by electrical inductance plethysmography. Because this technique provides only an approximate indication of respiratory variables, continuous measurement of end tidal carbon dioxide (CO2) was also done (Pilot, Colin Tonometry). Previous studies have shown that a face mask or mouthpiece introduce an artefactual increase in minute ventilation during spontaneous breathing.16 A more detailed respiratory evaluation obtainable by conventional equipment, using a mask or a mouthpiece, would thus have completely altered the patterns of breathing. The technique we used maintained more “spontaneous” conditions.
Data acquisition and analysis
All signals were digitised on-line by a 12 bit analogue to digital converter, as previously described.7,9,12 The RR interval, systolic and diastolic blood pressure, microcirculatory responses, respiration, and end tidal CO2 time series were obtained from these data. Any premature beats observed were interactively identified and corrected by linear interpolation with the previous and following beats. From our previous experience, an incidence of premature beats of up to 5% still enabled us to obtain an undistorted estimate of the LF and HF (high frequency) components. The original signals and time series were then stored for further analysis of each signal—including the mean signal, the signal variability (evaluated as the standard deviation), and autoregressive power spectrum analysis. The maximum fall in systolic or diastolic blood pressure was the lowest value observed during the entire period of tilting in relation to the mean supine value.
Power spectrum analysis
Power spectrum analysis was applied to all signals using an autoregressive model.6,7,9,14 The power spectrum of all signals except respiration shows at least two separate peaks, the higher frequency peak being similar in both shape and central frequency to respiration. Although in the RR interval sequence this peak seems mostly to reflect the parasympathetic efferent activity, in the circulatory signals it is interpreted mainly as being a mechanical consequence of respiration.6,7 This respiratory (high frequency) component is identified by its coincidence with the peak of the respiratory spectrum, which served as a reference. It has been suggested that the low frequency component (between 0.03–0.15 Hz, with its peak normally at around 0.10 Hz in the RR interval (LF)), when unrelated to any respiratory event, represents a marker of sympathetic activity,6–8 particularly in the blood pressure and the microcirculation.7,14 The LF and HF components of the RR interval were expressed in normalised units (NU) by expressing them as a percentage of total variability, after subtracting the power below the lower LF limit (that is, below 0.03 Hz).
Analysis of arterial baroreflex
Analysis of the arterial baroreflex was undertaken on the data obtained by spectral analysis.8,9 Briefly, the gain of the arterial baroreflex is obtained by dividing the fluctuations of the RR interval by the fluctuations of blood pressure at the same frequency.17 A mathematical function (the “squared coherence”) is used to ascertain that the fluctuations in these two signals at the same frequency are interrelated (coherence > 0.5); this requires that fluctuations in the RR interval are the result of the baroreflex response to similar (and related) fluctuations in blood pressure. Such an approach gives results comparable to those obtained using the Oxford phenylephrine test.8
Analysis of respiration
Because of the limitations described above, only simple essential respiratory variables could be evaluated during spontaneous breathing: breathing frequency was determined on the signal obtained by inductive belts, while end tidal CO2 was taken as an indirect index of hyperventilation, which allowed both intrasubject and intersubject comparisons.
Statistical analysis
The results are given as means (SEM). As the power spectral data of the various signals show a skewed distribution, descriptive statistics are calculated after natural logarithm transformation. Comparison of results was done using factorial or repeated measures analysis of variance and, if a significant (p < 0.05) overall difference was obtained, the Sheffé test was used to assess differences between the various signals. Linear regression analysis was used to assess the relation between different variables.
RESULTS
Examples of the signals and results obtained in one control and one amyloidotic subject are presented in figs 1 and 2. Data are presented in figs 3, 4, and 5.
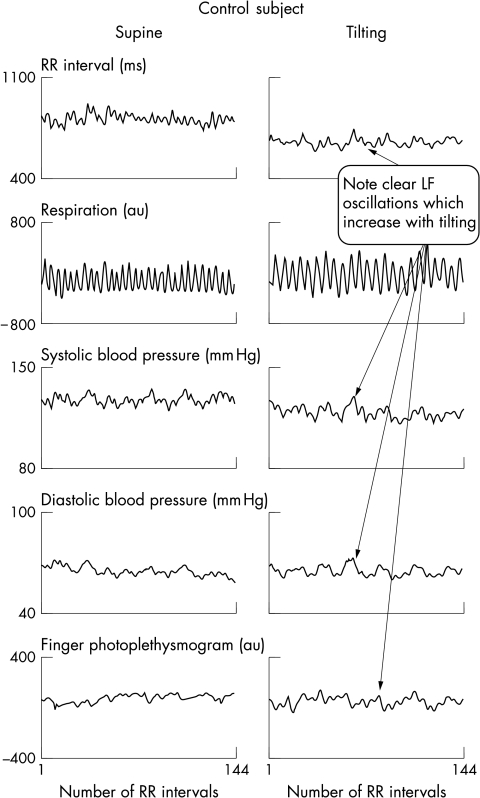
Figure 1.
Time series signals obtained in a control subject: supine position (left panels) and on tilting (right panels). Breathing frequency was controlled at 15 breaths/min to avoid spurious slow breathing (see Methods). Note that all cardiovascular signals show a variable proportion of fast fluctuations, similar to the respiratory rhythm, and slower fluctuations, clearly not related to respiration, which increase on tilting. These fluctuations are related to the activity of the sympathetic system.
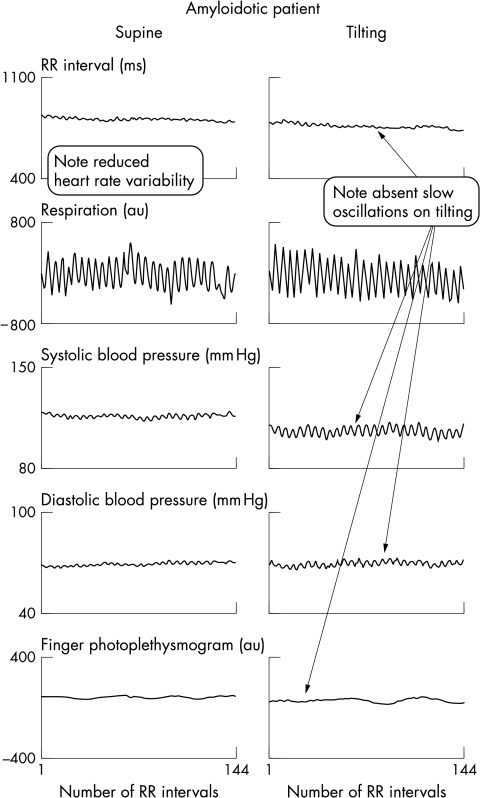
Figure 2.
Time series signals obtained in patient with primary amyloidosis: supine position (left panels) and on tilting (right panels). Breathing frequency was controlled at 15 breaths/min to avoid spurious slow breathing (see Methods). Note that, compared with fig 1, only fast (respiratory) fluctuations are evident in all signals. No changes are evident on tilting. Despite lack of sympathetic modulation, blood pressure levels remain unchanged upon tilting.
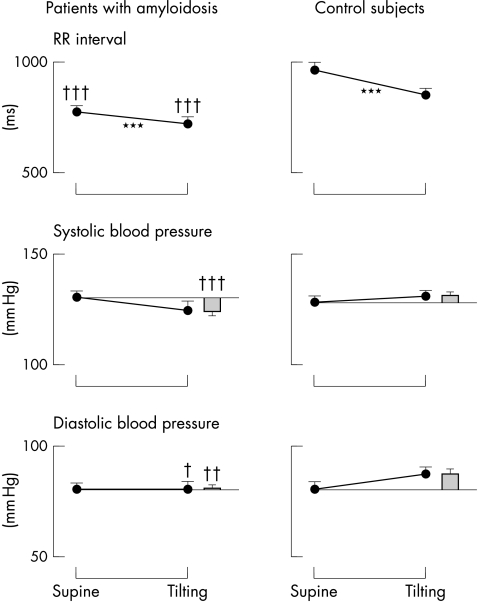
Figure 3.
Mean values of RR interval and systolic and diastolic blood pressures in subjects with primary amyloidosis and controls. The histograms indicate the difference in blood pressure resulting from tilting. ***p<0.001 v supine; †p<0.05, ††p<0.01, †††p<0.001 v controls. Note that despite a different trend, the average blood pressure values in the amyloid group remain similar to those found in the controls.
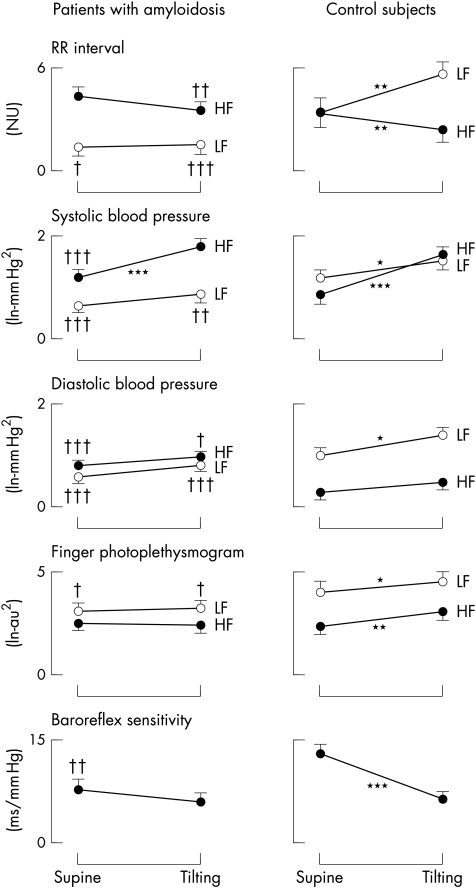
Figure 4.
Tilt induced trends of low (LF) and high (HF) frequency fluctuations in RR interval, systolic and diastolic blood pressure, microcirculation (assessed by finger photoplethysmography), and baroreflex sensitivity in subjects with primary amyloidosis and controls. *p<0.05, **p<0.01, ***p<0.001 v supine; †p<0.05, ††p<0.01, †††p<0.001 v controls. Note that all fluctuations caused by autonomic modulation (that is, the LF in all signals and the HF in the RR interval only) are notably reduced in the amyloid group. With tilting, the LF oscillations fail to increase in all signals in the amyloid group, in both absolute and relative terms. Note the greatly depressed baroreflex sensitivity in the amyloid group.
Figure 5.
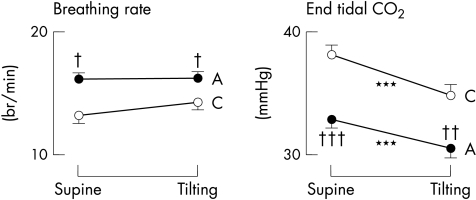
Breathing rate and end tidal CO2 in patients with primary amyloidosis (A) and controls (C). Note the higher breathing rate and the reduced end tidal CO2 in the amyloidotic group, indicating hyperventilation in these patients. ***p<0.001 v supine; †p<0.05, ††p<0.01, †††p<0.001 v controls.
RR interval
Autonomic modulation of heart rate (RR interval) was notably depressed in amyloidosis. Resting RR interval was shorter (fig 3), and both low and high frequency components of variability were, in absolute terms, notably reduced in the amyloidotic patients compared with the controls (LF power (mean (SEM)): 2.53 (0.32) v 4.89 (0.24) ln−ms2, respectively; p < 0.001; HF power: 3.43 (0.23) v 4.89 (0.20) ln−ms2; p < 0.001). Upon tilting, the normalised low frequency oscillations became predominant in controls, whereas no change was seen in the amyloid patients (fig 4); in contrast, the normalised high frequency components decreased greatly in the controls, but again showed no change in the amyloid group (fig 4). The LF/HF ratio in the supine position was notably depressed in the amyloid group with respect to the controls (1.27 (0.28) v 2.83 (0.90); p < 0.04) and did not increase significantly with tilting (to 2.31 (0.74), NS); the tilted value remained lower (p < 0.008) than in the controls, in whom it increased to 6.61 (1.67) (p < 0.05 v supine).
Systolic and diastolic blood pressures
Systolic and diastolic blood pressures were similar to controls, both when supine and on tilting. During tilting, the maximum fall in systolic and diastolic pressures was significantly greater in the amyloid patients (fig 3), but was well tolerated by all patients. Five amyloidotic subjects (but none of the controls) had a fall in systolic blood pressure of ≥ 25 mm Hg, but in only two subjects did it fall below 100 mm Hg, and then without major subjective distress. Despite these nearly normal blood pressure values, the autonomic modulation of both systolic and diastolic blood pressure, as assessed by the low frequency component of variability, was notably depressed in amyloidotic patients compared with the controls, both when supine and on tilting (fig 4). Furthermore, in contrast to the controls, tilting produced only a minimal and non-significant increase in the power of low frequency oscillations. Conversely, the respiratory fluctuations in blood pressure (both systolic and diastolic) were greater than in the controls, owing to the lack of buffering action exerted by changes in heart rate caused by the arterial baroreflex.
Autonomic modulation of skin microcirculation
Similarly to blood pressure, we found a pronounced reduction in the autonomic component of skin microvascular variability—that is, the low frequency component—whereas the mechanical (respiratory) component of variability was similar to the controls (fig 4).
Baroreflex sensitivity
In the supine position, baroreflex sensitivity was depressed in the amyloidotic patients compared with the controls; on tilting, baroreflex sensitivity decreased in the controls but not in the amyloid group. Although the values remained lower in the amyloidotic patients, the difference was no longer significant (fig 4).
Breathing abnormalities in amyloidosis
End tidal CO2 was consistently reduced in amyloidotic patients compared with the controls, both in the supine position and on tilting (fig 5). In addition, the amyloid group showed a higher breathing frequency (fig 5), which correlated inversely with the end tidal CO2 values (r = 0.28, p < 0.05 supine; and r = 0.29, p < 0.05 on tilting), indicating that subjects with amyloidosis were hyperventilating during spontaneous breathing, in both the supine and the tilted positions. CO2 concentrations did not correlate with either serum creatinine or 24 hour proteinuria.
Autonomic and laboratory correlates of the blood pressure fall on tilting
The fall in diastolic blood pressure on tilting was inversely correlated with:
baroreflex sensitivity (r = −0.39, p < 0.01 supine; r = −0.43, p < 0.01 tilting)
the power of low frequency oscillations in RR interval (r = −0.42, p < 0.01 supine; r = −0.43, p < 0.01 tilting)
the power of low frequency oscillations in systolic and diastolic blood pressures (systolic: r = −0.38, p < 0.025 supine, and r = 0.29, p < 0.05 tilting; diastolic: r = −0.33, p < 0.05 supine, and r = −0.30, p < 0.05 tilting)
the power of low frequency oscillations in finger photoplethysmogram (r = −0.31), p < 0.05 supine; r = −0.33, p < 0.05 tilting).
Thus, the subjects with less pronounced low frequency oscillations or lower baroreflex sensitivity showed a greater fall in diastolic blood pressure on tilting.
There was also a significant inverse correlation between the fall in diastolic blood pressure on tilting and the high frequency fluctuations in RR interval (supine: r = −0.40, p < 0.01 supine; tilting: r = −0.40, p < 0.01). The fall in diastolic blood pressure was inversely correlated with breathing rate (tilting: r = −0.29, p < 0.05); thus the subjects who maintained a slower breathing rate could tolerate the upright position better.
The fall in systolic blood pressure on tilting showed a similar trend, but the significance was in general less. However, there was a significant correlation with ventricular septal thickness (r = 0.39, p < 0.05). No systematic correlation was found between laboratory indices of renal function, packed cell volume, or plasma protein concentrations and either the blood pressure fall on tilting or the autonomic variables.
DISCUSSION
Our study shows that both vascular autonomic modulation and baroreflex function are affected by amyloidosis. While confirming that all indices of heart rate variability are depressed in primary amyloidosis, as in the familial form,4 we have also now found the following: autonomic (sympathetic) control to the blood vessels is impaired; the arterial baroreflex is notably impaired; and postural hypotension does not appear to be present in the majority of affected individuals, indicating that compensatory factors are operative until more advanced stages of the disease. Spontaneous hyperventilation might be one such compensatory factor.
Depression of heart rate variability in primary amyloidosis
In agreement with two previous reports,1,2 we confirm that heart rate variability is notably depressed in primary amyloidosis. The pattern of change indicates that both the parasympathetic component (respiratory sinus arrhythmia, here evaluated by the HF component of heart rate variability) and the sympathetic response to tilting (assessed by the lack of increase in the LF components of variability) are depressed in amyloidosis, in both absolute and relative terms. The reduction in the spectral parameters related to sympathetic activity (LF) correlated with the decrease in blood pressures on tilting. However, although the values found correlated weakly with the number of organs involved, with the progression of the disease, and with the time since diagnosis, the abnormality appears to be present from the time of initial diagnosis and also in patients who are free of clinical symptoms or cardiac involvement. This suggests that depressed heart rate variability may not be a crucial factor in the important debilitating symptom of postural hypotension.
Impairment of autonomic control of blood vessels in amyloidosis
Autonomic modulation of vascular reactivity is known to play a key role in maintaining driving pressure and venous return during the upright posture. We and others have previously shown that the low frequency modulation of blood pressure is important in maintaining blood pressure upon standing or passive tilting.18,19 Similarly, the LF components at a microvascular (arteriolar) level indicate that sympathetic activity modulates the resistance vessels. It is known that sympathetic modulation is notably reduced in other autonomic neuropathies associated with postural hypotension, such as diabetes.20,21 In the present study we found an extreme reduction in the autonomic modulation of both blood pressure and the microcirculation in amyloidosis. Although the power of the low frequency oscillations in these variables correlated with systolic and diastolic blood pressures, with the fall induced by tilting, and with the number of organs involved, it seems that other compensatory mechanisms may have reduced the effect of this almost total loss of sympathetic vascular modulation. The presence of left ventricular hypertrophy induced a further worsening of vascular function, as the systolic blood pressures on tilting were lower in subjects with increased septal thickness. Left ventricular hypertrophy may have reduced blood pressure on standing either by a reduction in stroke volume (diastolic dysfunction)22 or by a reduction in cardiopulmonary afferent traffic, as occurs in arterial hypertension.23
Impairment of the arterial baroreflex in amyloidosis
The arterial baroreflex is the most important physiological mechanism reacting to sudden alterations in blood pressure, such as occur during postural change. In our amyloid patients we found a notably depressed arterial baroreflex which was not related to a major disturbance of blood pressure in the upright position (although there was a significant correlation between baroreflex sensitivity and blood pressure fall on tilting). The arterial baroreflex is depressed after myocardial infarction and in chronic heart failure, and the greater the impairment the worse the prognosis.24 In patients with those conditions, however, the depressed baroreflex may be compensated for by an increase in sympathetic tone, as evidenced by high sympathetic nerve traffic and increased catecholamine levels,25 thus preventing the occurrence of postural hypotension until myocardial failure has become severe. Although we did not measure catecholamines in this study, it has been reported that they are decreased in patients with amyloid-associated autonomic neuropathy.26,27 Hypervolaemia might have blunted the effect of postural change, but the normal values of packed cell volume and serum proteins, and the observation that monoclonal protein was present at low concentration (< 10 g/l) in most of our patients (table 1), suggest that hypervolaemia was probably not present in our subjects.
Because postural hypotension did not appear to be present in most of our patients, it is possible that other compensatory factors remain operative until more advanced stages of the disease.
Hypothesis: is hyperventilation a compensatory factor in amyloidosis?
Our finding that amyloid patients show a spontaneous tendency to hyperventilate might provide an important insight into this problem. One of the effects of stimulating the low pressure baroreceptors present in the atria and the pulmonary vessels is to maintain the venous return; this is achieved both by an increase in sympathetic activity and—less well known—by an increase in ventilation, which acts to restore venous return by an increased effect of the thoracic pump.28 Owing to the evident failure of sympathetic modulation of the large and small arterial vessels in amyloidosis, hyperventilation may be the only effect of stimulation of the low pressure baroreceptors. In addition, direct stimulation of central chemosensitive areas could maintain hyperventilation even in the presence of neural afferent dysfunction. The inverse correlation found between breathing rate and pressure fall on tilting indicates that the increase in ventilation is more efficient at maintaining blood pressure if the breathing rate is slower; this is logical if one considers that a larger tidal volume (the necessary result of a lower respiratory rate combined with a greater minute ventilation29) produces a greater increase in venous return.
The importance of breathing as a compensatory mechanism in presyncopal episodes has recently been emphasised.10 In severely neuropathic patients (like those in the present study) this mechanism may be essential in helping to maintain the blood pressure on standing, and when this mechanism fails, postural hypotension is likely to ensue. In support of this hypothesis, three of the five patients with the greatest fall in blood pressure on tilting had normal end tidal CO2 concentrations with normal breathing rates, both supine and tilted, indicating an absence of hyperventilation; the other two subjects had low end tidal CO2 concentrations, but their breathing rates were also increased to ∼24 breaths/minute, thus nullifying the effect of hyperventilation. The reduced CO2 in our patients did not appear to be a compensatory response to renal acidosis—though renal involvement was present in more than half our patients—as there was no correlation between the CO2 values and either serum creatinine or 24 hour proteinuria.
Study limitations
The management of amyloidotic patients in the presence of cardiovascular failure is difficult. We therefore limited our investigations to simple baseline measurements, avoiding possibly disturbing tests such as neck suction or microneurography combined with vasoactive drug injections, even though these would have defined the function of the vascular efferent branch of the arterial baroreflex. The almost complete absence of autonomic modulation of both heart rate and vascular reactivity, combined with the pronounced baroreflex impairment, suggests that non-pharmacological interventions such as neck suction should be relatively safe (similar to the situation after heart transplantation30); this will be a matter for future investigation. For the same reasons of safety, we did not attempt to undertake respiratory challenge by alterations in blood gases. We did not do any catecholamine measurements in the present study, but previous reports clearly indicate a reduced catecholamine response to tilting in amyloid patients. We did not attempt to assess the presence of renal acidosis, but the simple measurements of renal function which were done (serum creatinine and urea) did not correlate at all with the extent of hyperventilation. This suggests that the hyperventilation was not a response to renal involvement.
Clinical implications and conclusions
Primary amyloidosis causes a generalised abnormality of neural autonomic control of heart rate and vascular reactivity; nevertheless, the blood pressure remains normal in the early phase of the disease, suggesting the presence of additional compensatory mechanisms—including hyperventilation to increase venous return in the upright posture. We suggest that failure to hyperventilate, or hyperventilation at a high respiratory rate, is likely to precipitate postural hypotension and a rapid deterioration in the patient’s clinical condition. These results have clinical relevance for our understanding of the pathophysiology of amyloidosis, and also for the clinical diagnosis and staging of autonomic involvement in this disease. Furthermore, the data provide the basis for practical, non-pharmacological management to allow partial compensation for postural hypotension in those patients who are affected by postural hypotension.10,11 Finally, as the impact of breathing patterns on other types of postural hypotension (a common and debilitating condition, particularly in elderly people and in patients with diabetes) has not yet been investigated, the implications of this study may extend to other more common forms of postural hypotension.
Supplementary Material
Acknowledgments
This study was supported by a grant from European Community (Biomed 2 Program No BMH4-CT 98-3689); by Telethon Italy; and by a grant from IRCCS Policlinico S Matteo, Pavia, Italy. Presented in part in abstract form at the 72nd American Heart Association Scientific Sessions, Atlanta, Nov 7–10, 1999 (Circulation 1999;100:I-133).
REFERENCES
- 1.Berg AM, Anderson JJ, Chipkin SR, et al. Autonomic neuropathy in AL (primary) amyloidosis and its effect on survival. Amyloid Int J Exp Clin Invest 1994;1:39–46. [Google Scholar]
- 2.Gemmi F, Miliani A, Bergesio F, et al. Functional study of autonomic neuropathy in primary (AL) amyloidosis. Amyloid Int J Exp Clin Invest 1996;3:167–72. [Google Scholar]
- 3.Kinoshita O, Hongo M, Saikawa Y, et al. Heart rate variability in patients with familial amyloid polyneuropathy. PACE 1997; 20:2949–53. [DOI] [PubMed] [Google Scholar]
- 4.Ando Y, Suhr OB. Autonomic dysfunction in familial amyloidotic polyneuropathy (FAP). Amyloid Int J Exp Clin Invest 1998;5:288–300. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho MJ, Man in’t Veld AJ, Costa O, et al. Spectral analysis of the heart rate as an assessment of autonomic function in familial amyloid polyneuropathy. J Hypertens 1991;9(suppl 6):S62–3. [PubMed] [Google Scholar]
- 6.Malliani A , Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84: 482–92. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi L, Hayoz D, Wenzel R, et al. Synchronous and baroreceptor-sensitive oscillations in skin microcirculation. Evidence for central autonomic control. Am J Physiol 1997;42:1867–78. [DOI] [PubMed] [Google Scholar]
- 8.Pagani M, Somers V, Furlan R, et al. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension 1988;12:600–10. [DOI] [PubMed] [Google Scholar]
- 9.Radaelli A, Bernardi L, Valle F, et al. Cardiovascular autonomic modulation in essential hypertension: effect of tilting. Hypertension 1994;24:556–63. [DOI] [PubMed] [Google Scholar]
- 10.Lipsitz LA, Hayano J, Sakata S, et al. Complex demodulation of cardiorespiratory dynamics preceding vasovagal syncope. Circulation 1998;98:977–83. [DOI] [PubMed] [Google Scholar]
- 11.Van Lieshout JJ, Ten Harkel ADJ, Wieling W. Physical manoeuvres for combating orthostatic dizziness in autonomic failure. Lancet 1992;339:897–8. [DOI] [PubMed] [Google Scholar]
- 12.Perfetti V, Garini P, Colli Vignarelli M, et al. Diagnostic approach to and follow-up of difficult cases of AL amyloidosis. Haematologica 1995;80:409–15. [PubMed] [Google Scholar]
- 13.Kemmotsu O, Ueda M, Otsuka H, et al. Arterial tonometry for noninvasive, continuous blood pressure monitoring during anesthesia. Anesthesiology 1991;75:333–40. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi L, Radaelli A, Soldá PL, et al. Autonomic control of skin microvessels assessment by power spectrum of photoplethysmographic waves. Clin Sci 1996;90:345–55. [DOI] [PubMed] [Google Scholar]
- 15.Bernardi L, Leuzzi S. Laser Doppler velocimetry and photoplethysmography: hardware and measuring principles. In: Berardesca E, Elsner P, Maibach H, eds. Handbook of skin engineering, vol 2, Boca Raton: CRC Press, 1994:31–56.
- 16.Askanazi J, Silverberg PA, Foster RJ, et al. Effects of respiratory apparatus on breathing pattern. J Appl Physiol 1980;48:577–80. [DOI] [PubMed] [Google Scholar]
- 17.Baselli G, Cerutti S, Civardi S, et al. Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Comput Biomed Res 1986;19:520–34. [DOI] [PubMed] [Google Scholar]
- 18.Ten Harkel AD, Van Lieshout JJ, Karemaker JM, et al. Differences in circulatory control in normal subjects who faint and who do not faint during orthostatic stress. Clin Auton Res 1993;3:117–24. [DOI] [PubMed] [Google Scholar]
- 19.Bernardi L, Salvucci F, Leuzzi S, et al. Cardiovascular reflex changes preceding episodes of vasovagal syncope in paediatric subjects. Clin Sci 1996;91:25–7. [DOI] [PubMed] [Google Scholar]
- 20.Hilsted J. Decreased sympathetic vasomotor tone in diabetic orthostatic hypotension. Diabetes 1979;28:970–3. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi L, Rossi M, Leuzzi S, et al. Reduction of 0.1 Hz microcirculatory fluctuations as an evidence of sympathetic dysfunction in insulin-dependent diabetes. Cardiovasc Res 1997;34:185–91. [DOI] [PubMed] [Google Scholar]
- 22.Chamarthi B, Dubrey SW, Cha K, et al. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol 1997;80:1242–5. [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Giannattasio C, Cleroux J, et al. Cardiopulmonary reflex before and after regression of left ventricular hypertrophy in essential hypertension. Hypertension 1988;12:227–37. [DOI] [PubMed] [Google Scholar]
- 24.LaRovere MT, Bigger JT, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–84. [DOI] [PubMed] [Google Scholar]
- 25.Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 1995;92:3206–11. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Tsuge I, Higa S, et al. Catecholamine metabolism in familial amyloid polyneuropathy. Clin Genet 1979;16:117–24. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein AE, Yahr MO, Mytileneou C, et al. Peripheral catecholamine depletion in amyloid autonomic neuropathy. Mt Sinai J Med 1978;45:782–9. [PubMed] [Google Scholar]
- 28.Bevan JA. The pulmonary artery baroreceptor region. Baroreceptors and hypertension. Oxford: Pergamon Press, 1967: 69–5.
- 29.Bernardi L, Spadacini G, Bellwon J, et al. Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 1998;351:1308–11. [DOI] [PubMed] [Google Scholar]
- 30.Bernardi L, Bianchini B, Spadacini G, et al. Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval. Circulation 1995;92:2895–903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.