Abstract
Background: Glucose–insulin–potassium (GIK) infusion improves cardiac function and outcome during acute ischaemia.
Objective: To determine whether GIK infusion benefits patients with chronic ischaemic left ventricular dysfunction, and if so whether this is related to the presence and nature of viable myocardium.
Methods: 30 patients with chronic ischaemic left ventricular dysfunction had dobutamine echocardiography and were given a four hour infusion of GIK. Segmental responses were quantified by improvement in wall motion score index (WMSI) and peak systolic velocity using tissue Doppler. Global responses were assessed by left ventricular volume and ejection fraction, measured using a three dimensional reconstruction. Myocardial perfusion was determined in 15 patients using contrast echocardiography.
Results: WMSI (mean (SD)) improved with dobutamine (from 1.8 (0.4) to 1.6 (0.4), p < 0.001) and with GIK (from 1.8 (0.4) to 1.7 (0.4), p < 0.001); there was a similar increment for both. Improvement in wall motion score with GIK was observed in 55% of the 62 segments classed as viable by dobutamine echocardiography, and in 5% of 162 classed as non-viable. There was an increment in peak systolic velocity after both dobutamine echocardiography (from 2.5 (1.8) to 3.2 (2.2) cm/s, p < 0.01) and GIK (from 3.0 (1.6) to 3.5 (1.7) cm/s, p < 0.001). The GIK effects were not mediated by changes in pulse, mean arterial pressure, lactate, or catecholamines, nor did they correlate with myocardial perfusion. End systolic volume improved after GIK (p = 0.03), but only in 25 patients who had viable myocardium on dobutamine echocardiography.
Conclusions: In patients with viable myocardium and chronic left ventricular dysfunction, GIK improves wall motion score, myocardial velocity, and end systolic volume, independent of effects on haemodynamics or catecholamines. The response to GIK is observed in areas of normal and abnormal perfusion assessed by contrast echocardiography.
Keywords: ischaemic heart disease, glucose-insulin-potassium infusion, dobutamine echocardiography
Left ventricular dysfunction is a major determinant of outcome in coronary artery disease.1 However, not all dyssynergic areas are irreversibly damaged after infarction, as left ventricular dysfunction may be caused by myocardial stunning2 or by chronic reduction of blood flow,3 and the segments involved may show improved function after restoration of blood flow.4 Preservation of metabolism despite reduced function is the cornerstone of viable myocardium, but ultrastructural changes have been documented in viable tissue, including loss of sarcoplasmic reticulum, small scattered mitochondria, and glycogen deposition.5 These changes suggest a reduction in aerobic metabolism, but glucose uptake and utilisation are preserved and indeed have been used as a diagnostic marker of viability.6
Because of co-morbidity, myocardial revascularisation may be difficult to achieve in many patients with left ventricular dysfunction and viable myocardium. In these situations, medical treatment to improve left ventricular function may be useful in the short or intermediate term. Infusion of glucose, insulin, and potassium (GIK) has been shown to attenuate ischaemic injury and reduce post-ischaemic dysfunction7—for example, at the time of cardiopulmonary bypass8 or valve replacement.9 In the setting of acute myocardial infarction, GIK infusions appear to reduce infarct size and enhance functional recovery.10,11 Early after infarction, GIK has been shown to improve exercise tolerance, to decrease ST segment shift, and to improve wall motion abnormalities.12 Although the clinical value of these metabolic interventions was undefined for some time, recent studies suggest a mortality reduction similar to that with thrombolytic treatment.13 In chronic coronary artery disease, the impact of metabolic interventions on left ventricular function is not clear, although recent studies of trimetazidene have suggested that improvement in both regional14 and global left ventricular function15 is possible. Our aim in this study was to assess the effect of GIK on global and regional myocardium, and its association with viability, in the setting of chronic ischaemic left ventricular dysfunction.
METHODS
Study population
The study involved 30 patients with previous myocardial infarction, resting wall motion abnormalities, and left ventricular dysfunction. All patients were on standard medical treatment. Patients underwent dobutamine stress echocardiography and were recalled for a four hour infusion of GIK. Regional wall motion was assessed qualitatively by wall motion scoring and quantitatively by tissue Doppler velocity. Left ventricular volumes and ejection fraction were compared before and after each infusion using three dimensional reconstruction of the left ventricle.
Stimulation of left ventricular function
Dobutamine echocardiography
A standard dobutamine stress protocol was used, involving infusion of incremental doses of dobutamine (5–40 μg/kg/min), with atropine (to a total of 2 mg intravenously) if 85% peak heart rate was not attained at peak dobutamine dose.16 β Blockers were withheld on the day of the test. Patients were monitored in accordance with the standard protocol. Standard end points were used: conclusion of the protocol; development of severe angina or other intolerable symptoms; development of hypertension (systolic pressure > 230 mm Hg); symptomatic hypotension; serious arrhythmia; or extensive ischaemia. Images obtained in standard views at rest, with low dose dobutamine, and after peak stress were compared using a quad screen display.
Glucose–insulin–potassium infusion
Patients were recalled for a four hour infusion of GIK. Oral hypoglycaemic agents were withheld on the day of GIK infusion. After insertion of a peripheral venous cannula, blood was taken for measurement of baseline glucose, lactate, electrolytes, and serum catecholamines. Baseline blood pressure and pulse rate were recorded and patients underwent resting echocardiography, including tissue Doppler imaging. They then received a four hour infusion of GIK (10% dextrose, 80 U insulin, 40 mmol KCl) at 30 ml/h. The GIK infusion used was similar to that used in the DIGAMI (diabetes mellitus insulin–glucose infusion in acute myocardial infarction) study,17 with the exception that our infusion consisted of 10% dextrose to minimise hypoglycaemic episodes. Blood glucose was checked hourly, and patients were given supplemental 50% glucose intravenously if their blood glucose fell below 4.0 mmol/l in order to avoid symptomatic hypoglycaemia. After four hours of GIK, the infusion was stopped and blood samples and echocardiography, including tissue Doppler imaging, were repeated.
Analysis of regional function
Radial function
Wall motion data on the dobutamine echocardiography and GIK studies were scored separately and independently by a blinded investigator, using the 16 segment model of the American Society of Echocardiography.18 Regional myocardial performance was scored using a four point scale: 1, normal; 2, hypokinetic; 3, akinetic; 4, dyskinetic. A wall motion score index (WMSI) was calculated by adding the numerical value assigned to each segment and dividing by the number of segments visualised. Segments with severe resting hypokinesis or akinesis were identified as scar, except when regional function improved with low dose dobutamine, in which case they were identified as viable. Ischaemia was identified by new or worsening wall motion abnormalities with dobutamine stress; a biphasic response was identified if improvement at low dose was followed by deterioration at peak doses of dobutamine.
Longitudinal (base–apex) function
Colour tissue Doppler data were saved in each view at rest, at low dose dobutamine, and before and after GIK in each patient. Colour Doppler frame rates ranged from 80–115 frames/s depending on the sector width of the range of interest, and aliasing velocities were selected between 16–32 cm/s. Digital storage of three cardiac cycle loops triggered to the QRS complex were saved to magneto-optical disk for off-line analysis. Colour Doppler cine loops acquired were analysed using standard software (Echopac version 6.1, General Electric-Vingmed, Milwaukee, Wisconsin, USA). Maximum base to apex axis systolic velocities were obtained by location of the sample volume in the middle of each segment at peak stress. Myocardial velocities were measured off-line in all myocardial segments except the apical ones, which have been found to have a high noise to signal ratio.19
Contrast echocardiography
Images were obtained using a standard echocardiography machine (HDI5000, Advanced Technology Laboratories, Bothell, Washington, USA) with a P4-2 ultrasound probe; all contrast imaging was done under resting conditions. Acquisitions involved triggered grey scale imaging and triggered harmonic power Doppler, using end systolic 1:4, 1:6, and 1:8 intervals and a mechanical index of 1.3. Digitised baseline and contrast grey scale images at each triggering interval were averaged and the baseline images digitally subtracted from the contrast images. The resulting image was then colour coded using a heated object algorithm as previously described.20 A blinded experienced observer scored myocardial perfusion in each segment from the apical view by review of both power Doppler and colour coded images. Segments were scored as 2 (normal perfusion), 1 (reduced perfusion), or 0 (no perfusion).
Analysis of global function
Left ventricular volumes were measured after three dimensional reconstruction from the cross sectional images at baseline and after GIK, using an approach previously shown to be accurate and reproducible.21 End systolic and end diastolic endocardial contours were traced off line from digitally stored resting images in each view. End systolic volume was measured on the frame immediately following isovolumic contraction; end diastolic volume was measured as the frame with the largest cardiac blood volume. The ejection fraction was calculated from these volumes in the usual fashion.
Statistical analysis
All variables shown are mean (SD) or frequency and percentage, unless otherwise stated. Continuous variables were compared with the Student’s t test if data were normally distributed and the Wilcoxon rank-sum test if they were not. Baseline clinical characteristics of the two groups were compared with two tailed independent t tests for continuous variables and the χ2 tests for non-continuous variables. Differences were considered significant at probability values of p < 0.05. Data analysis was done with SPSS statistical software, version 9 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Baseline demographics
The subjects were 27 men and three women, mean (SD) age 65 (9) years. Most of them were obese, with a mean body mass index of 29.0 (3.8) kg/m2. There were nine subjects with non-insulin dependent diabetes, of whom one required insulin. Other risk factors included current smoking (3), ex-smoking (20), hypercholesterolaemia (25), and hypertension requiring treatment (19). Seven patients had previous coronary artery bypass surgery and one had previous angioplasty. The median New York Heart Association functional class was II, and the median Canadian Heart Association status was 1. Patients were on standard treatment for left ventricular dysfunction and coronary artery disease. Angiotensin converting enzyme inhibitors were being used in 21 patients (70%) and β adrenoceptor blocking agents in 19 (63%). Most of the patients were on antiplatelet treatment (97%) and statins (82%).
GIK infusion
Patients received 108 (18) ml of GIK, providing 17 (3) units of insulin, over 3.9 (0.4) hours. The haemodynamic and biochemical responses to GIK infusion are summarised in table 1. Nineteen patients required supplemental intravenous boluses of 50% glucose to keep their blood glucose above 4.0 mmol/l, and eight of these patients reported symptoms of hypoglycaemia. Most biochemical variables, including lactate and noradrenaline (norepinephrine), remained unchanged. Although there was a significant increase in serum adrenaline (epinephrine) concentrations, the average concentration of adrenaline remained within the normal range, and the increase reflected two patients with a body mass index of < 26 kg/m2 who had recurrent episodes of hypoglycaemia and a more than fourfold increases in serum adrenaline. When these two patients were removed from the analysis there was no significant change in serum adrenaline response. The adrenaline concentration was not significantly different in patients who had symptoms of hypoglycaemia and those who did not, and there was no correlation between blood glucose and change in adrenaline.
Table 1.
Haemodynamic and biochemical changes with glucose–insulin–potassium (GIK)
| Pre-GIK | Post-GIK | p Value | |
| Pulse (beats/min) | 64 (9) | 62 (9) | NS |
| Mean arterial pressure (mm Hg) | 88 (13) | 87 (15) | NS |
| Glucose (mmol/l) (3.0–7.8)* | 7.8 (3.0) | 5.6 (2.7) | <0.001 |
| Potassium (mmol/l) (3.2–4.5)* | 4.1 (0.4) | 3.8 (0.4) | <0.001 |
| Adrenaline (nmol/l) (0.1–1.5)* | 0.3 (0.2) | 0.4 (0.4) | 0.03 |
| Noradrenaline (nmol/l) (0.1–6.3)* | 1.5 (0.8) | 1.6 (1.2) | NS |
| Lactate (mmol/l) (0.7–2.5)* | 1.6 (0.5) | 1.5 (4.0) | NS |
Values are mean (SD).
*Normal range in parentheses.
Wall motion scoring
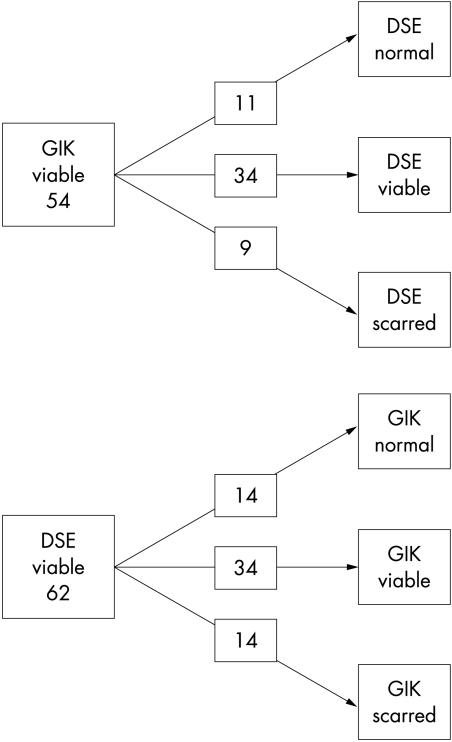
Wall motion scores with dobutamine echocardiography and GIK are summarised in table 2. The results of dobutamine echocardiography identified 256 segments as normal, 62 as viable, and 162 as infarcted. WMSI improved with both dobutamine (from 1.78 (0.4) to 1.64 (0.4), p < 0.001) and GIK (from 1.76 (0.4) to 1.66 (0.4), p < 0.001), and the improvement increment was similar with dobutamine echocardiography and GIK. There was no correlation between change in catecholamines and the magnitude of change in WMSI with GIK. Not all segments that improved with dobutamine showed an improvement with GIK (fig 1); GIK improved function in 55% of the 62 segments showing viability by dobutamine echocardiography. However, GIK led to improvement in only 5% of the 162 segments showing no contractile reserve to dobutamine.
Table 2.
Segmental responses to dobutamine echocardiography (DSE) and glucose–insulin–potassium infusion (GIK)
| DSE | GIK | |
| Normal | 256 | 261 |
| Viable | 62 | 54 |
| Scarred | 162 | 165 |
Figure 1.
Responses of viable myocardium to glucose-insulin-potassium (GIK) and dobutamine. DSE, dobutamine stress echocardiography.
Quantitation of regional function
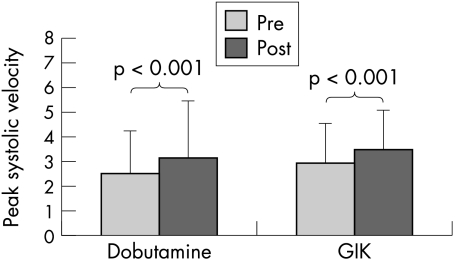
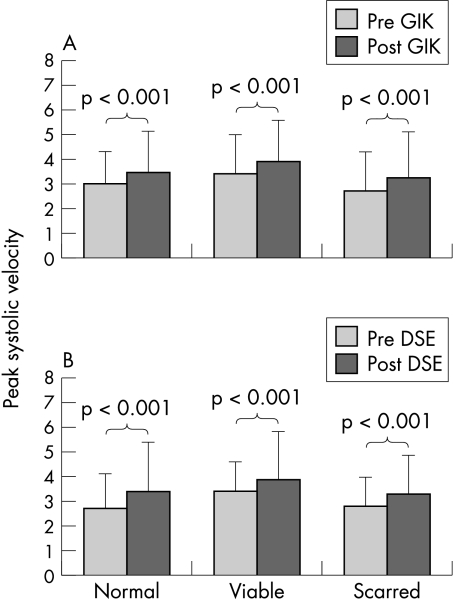
The longitudinal functional responses to dobutamine echocardiography and GIK are summarised in figs 2 and 3. Peak systolic velocity was similar at baseline and peak with dobutamine echocardiography and GIK. Figure 2 shows the significant increment in peak systolic velocity after both dobutamine echocardiography (from 2.5 to 3.1 cm/s, p < 0.01), and GIK (from 3.0 to 3.5 cm/s, p < 0.001); the increase with dobutamine echocardiography was not significantly different from the increase with GIK. Normal, viable, and scarred tissue all appeared to show increased velocity with both GIK and dobutamine echocardiography (fig 3).
Figure 2.
Peak systolic velocity before and after low dose dobutamine and glucose–insulin–potassium (GIK).
Figure 3.
Peak systolic velocity at rest and (A) after glucose–insulin–-potassium (GIK) and (B) dobutamine (DSE) in segments classified as normal, viable, and scarred.
Myocardial contrast echocardiography
In the 15 patients who underwent myocardial contrast echocardiography there were 25 viable segments on dobutamine echocardiography, of which 17 showed evidence of perfusion. There was no significant difference in myocardial perfusion between the segments that responded to GIK and those that did not (mean perfusion 0.86 (0.9) v 0.68 (0.8), NS).
Left ventricular volumes and ejection fraction
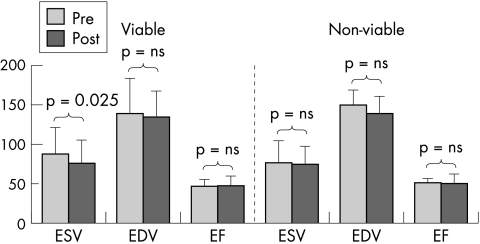
End systolic volume improved after GIK (p = 0.026) without change in end diastolic volume or ejection fraction. The improvement in end systolic volume was confined to 25 patients who responded to GIK (table 3). Similarly, only patients with echocardiographically improved wall motion score with dobutamine showed a change in end systolic volume (12 ml, p = 0.016) (fig 4). There was no correlation between the extent of change in end systolic volume and the magnitude of change in catecholamines.
Table 3.
Changes in end systolic volume related to response to glucose-insulin-potassium infusion (GIK)
| GIK responsive | GIK unresponsive | |||||
| Pre | Post | p Value | Pre | Post | p Value | |
| End systolic volume (ml) | 85 (36) | 72 (29) | 0.016 | 86 (10) | 87 (11) | NS |
| End diastolic volume (ml) | 134 (38) | 132 (38) | NS | 157 (22) | 145 (22) | NS |
| Ejection fraction (%) | 45 (9)% | 48 (11)% | NS | 48 (5)% | 48 (10)% | NS |
Values are mean (SD).
Pre/post, before/after GIK infusion.
Figure 4.
Changes in volumes with glucose–insulin–potassium according to viable tissue. ESV, end systolic volume; EDV, end diastolic volume; EF, ejection fraction.
DISCUSSION
Our results suggest that reduced regional left ventricular function in patients with previous myocardial infarction may be improved by giving GIK, and that the degree of improvement is similar to that achievable with dobutamine. The response of viable segments to GIK appears to be independent of perfusion on contrast echocardiography, suggesting that both stunned and hibernating tissue respond to GIK. Moreover, global left ventricular function can be improved with GIK in patients with viable myocardium. Thus it appears that in chronic left ventricular dysfunction, the performance of myocardium showing contractile reserve can be improved by enhancing the substrate supply.
Myocardial viability
In the setting of coronary artery disease, prolonged contractile dysfunction in non-infarcted myocardium is thought either to reflect post-ischaemic dysfunction (myocardial stunning)22 or to be an adaptation to chronic hypoperfusion (hibernation).3 In both situations, myocardial function may improve with normalisation of the oxygen supply:demand ratio. Although there is general agreement in published reports regarding the pathophysiology of stunned myocardium, the origin of myocardial hibernation remains uncertain, though affected myocardium clearly remains metabolically active.23
Possible mechanisms of action of GIK
The possible mechanisms of action of GIK include metabolic effects, direct haemodynamic effects, improvements of coronary flow, and catecholamine mediated effects.
Metabolic effects
The main source of the beneficial effects of GIK infusion is likely to be metabolic. Because of the local effects of insulin and through its suppression of free fatty acids, insulin infusions cause an increase in glucose uptake in both normal and viable myocardium.24,25 Insulin infusions also encourage a greater rate of ATP production from glycolysis.7 In the resting fasting state glucose only accounts for about 30% of the heart’s energy production; suppression of free fatty acids by insulin allows the myocardium to increase the utilisation of glucose, which is a more efficient energy source.26 It might be anticipated that both stunned and hibernating myocardium could show responsiveness to GIK infusion. Hibernating myocardium—which is chronically malperfused—may improve simply because of delivery of more substrate to the tissue. Stunned myocardium has normal perfusion, but may be more sensitive than usual to the effects of insulin because of upregulation of glucose transporter molecules,27 and increased ATP may augment function by restoring calcium homeostasis.
Direct haemodynamic effects
The beneficial effect of GIK is felt by some to reflect the haemodynamic effects of insulin.28 Certainly, insulin (either exogenous or endogenous, in response to an oral glucose load) has been shown to improve the left ventricular ejection fraction in normal subjects and in patients after myocardial infarction. Infusion of GIK has been shown to augment the exercise induced increase in ejection fraction in both healthy subjects and diabetic patients, although less so in the diabetic group.29 However, although insulin has a positive inotropic effect in cats and lambs, this has not been observed in other mammals, including humans.29
Improvement in coronary flow
A third mechanism might include improvement in myocardial perfusion, although this would not account for improvement in stunned myocardium. Coronary hyperinsulinaemia has been shown to increase coronary blood flow in patients with significant coronary artery disease.30 This vasodilatation may, at least in part, be responsible for the improvement in hibernating regions with GIK infusion.
Catecholamine mediated effects
Finally, the effects of GIK infusion may be mediated by concomitant changes in catecholamine concentrations. Insulin induced hypoglycaemia only has a small effect on serum noradrenaline31 but causes a pronounced increase in adrenaline.32 Insulin activates sympathetic nerves in the absence of hypoglycaemia.33 However, while the change in serum adrenaline was significant in this study, it appeared to reflect two individuals who had recurrent hypoglycaemia, and the findings do not explain the functional changes observed in the study. The lack of correlation between serum adrenaline and changes in WMSI and end systolic volume imply that the benefit of GIK was independent of serum catecholamine concentrations. Moreover, the lack of correlation between blood sugar and adrenaline concentrations in this study probably reflects correction of blood sugar with the onset of symptoms.
Clinical implications
GIK appears to improve regional and global cardiac function, especially in patients with viable myocardium. While a GIK infusion has limited feasibility, further studies on the time course and duration of this effect would be of value. Our results suggest that increasing the supply of myocardial substrate is safe when given in a controlled environment; symptoms of hypoglycaemia were easily corrected with intravenous glucose. Metabolic treatments for viable myocardium may be useful in patients in whom revascularisation is not possible because of co-morbidity or for technical reasons.
Acknowledgments
Supported in part by the National Health and Medical Research Council, Canberra, Australia. VKK is the holder of a CVL award, Sydney, Australia.
Abbreviations
DIGAMI, diabetes mellitus insuling
glucose infusion in acute myocardial infarction; GIK, glucose
insulin
potassium; WMSI, wall motion score index
REFERENCES
- 1.Alderman EL, Fisher LD, Litwin P, et al. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation 1983;68:785–95. [DOI] [PubMed] [Google Scholar]
- 2.Bolli R. Myocardial “stunning” in man. Circulation 1992;86:1671–91. [DOI] [PubMed] [Google Scholar]
- 3.Rahimtoola SH. The hibernating myocardium. Am Heart J 1989;117:211–21. [DOI] [PubMed] [Google Scholar]
- 4.Diamond GA, Forrester JS, deLuz PL, et al. Post-extrasystolic potentiation of ischemic myocardium by atrial stimulation. Am Heart J 1978;95:204–9. [DOI] [PubMed] [Google Scholar]
- 5.Vanoverschelde JL, Melin JA. The pathophysiology of myocardial hibernation: current controversies and future directions. Prog Cardiovasc Dis 2001;43:387–98. [DOI] [PubMed] [Google Scholar]
- 6.Tillisch J, Brunken R, Marshall R, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 1986;314:884–8. [DOI] [PubMed] [Google Scholar]
- 7.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 1999;99:578–88. [DOI] [PubMed] [Google Scholar]
- 8.Lazar HL, Philippides G, Fitzgerald C, et al. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. J Thorac Cardiovasc Surg 1997;113:354–60. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield GS, Commerford PJ, Opie LH. Effects of preoperative glucose-insulin-potassium on myocardial glycogen levels and on complications of mitral valve replacement. J Thorac Cardiovasc Surg 1986;91:874–8. [PubMed] [Google Scholar]
- 10.Ahmed SS, Lee CH, Oldewurtel HA, et al. Sustained effect of glucose-insulin-potassium on myocardial performance during regional ischemia. Role of free fatty acid and osmolality. J Clin Invest 1978;61:1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opie LH, Bruyneel K, Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation 1975;52:49–57. [DOI] [PubMed] [Google Scholar]
- 12.Marano L, Bestetti A, Lomuscio A, et al. Effects of infusion of glucose-insulin-potassium on myocardial function after a recent myocardial infarction. Acta Cardiol 2000;55:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Apstein CS, Taegtmeyer H. Glucose-insulin-potassium in acute myocardial infarction: the time has come for a large, prospective trial. Circulation 1997;96:1074–7. [DOI] [PubMed] [Google Scholar]
- 14.Lu C, Dabrowski P, Fragasso G, et al. Effects of trimetazidine on ischemic left ventricular dysfunction in patients with coronary artery disease. Am J Cardiol 1998;82:898–901. [DOI] [PubMed] [Google Scholar]
- 15.Brottier L, Barat JL, Combe C, et al. Therapeutic value of a cardioprotective agent in patients with severe ischaemic cardiomyopathy. Eur Heart J 1990;11:207–12. [DOI] [PubMed] [Google Scholar]
- 16.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol 1997;30:595–606. [DOI] [PubMed] [Google Scholar]
- 17.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65. [DOI] [PubMed] [Google Scholar]
- 18.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- 19.Fathi R, Cain P, Nakatani S, et al. Effect of tissue Doppler on the accuracy of novice and expert interpreters of dobutamine echocardiography. Am J Cardiol 2001;88:400–5. [DOI] [PubMed] [Google Scholar]
- 20.Haluska B, Case C, Short L, et al. Effect of power Doppler and digital subtraction techniques on the comparison of myocardial contrast echocardiography with SPECT. Heart 2001;85:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aakhus S, Maehle J, Bjoernstad K. A new method for echocardiographic computerized three-dimensional reconstruction of left ventricular endocardial surface: in vitro accuracy and clinical repeatability of volumes. J Am Soc Echocardiogr 1994;7:571–81. [DOI] [PubMed] [Google Scholar]
- 22.Braunwald E, Kloner RA. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation 1982;66:1146–9. [DOI] [PubMed] [Google Scholar]
- 23.Vanoverschelde JL, Wijns W, Borgers M, et al. Chronic myocardial hibernation in humans. From bedside to bench. Circulation 1997;95:1961–71. [DOI] [PubMed] [Google Scholar]
- 24.Maki M, Luotolahti M, Nuutila P, et al. Glucose uptake in the chronically dysfunctional but viable myocardium. Circulation 1996;93:1658–66. [DOI] [PubMed] [Google Scholar]
- 25.McNulty PH. Comparison of local and systemic effects of insulin on myocardial glucose extraction in ischemic heart disease. Am J Physiol (Heart Circ Physiol) 2000;278:H741–7. [DOI] [PubMed] [Google Scholar]
- 26.Wallhaus TR, Taylor M, DeGrado TR, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation 2001;103:2441–6. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto K, Nishimura T, Ishikawa M, et al. Enhancement of glucose uptake in stunned myocardium: role of glucose transporter. Am J Physiol 1997;272:H1122–30. [DOI] [PubMed] [Google Scholar]
- 28.Parsonage WA, Hetmanski D, Cowley AJ. Beneficial haemodynamic effects of insulin in chronic heart failure. Heart 2001;85:508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasso FC, Carbonara O, Cozzolino D, et al. Effects of insulin-glucose infusion on left ventricular function at rest and during dynamic exercise in healthy subjects and noninsulin dependent diabetic patients: a radionuclide ventriculographic study. J Am Coll Cardiol 2000;36:219–26. [DOI] [PubMed] [Google Scholar]
- 30.McNulty PH, Pfau S, Deckelbaum LI. Effect of plasma insulin level on myocardial blood flow and its mechanism of action. Am J Cardiol 2000;85:161–5. [DOI] [PubMed] [Google Scholar]
- 31.Fagius J, Niklasson F, Berne C. Sympathetic outflow in human muscle nerves increases during hypoglycemia. Diabetes 1986;35:1124–9. [DOI] [PubMed] [Google Scholar]
- 32.Garber AJ, Cryer PE, Santiago JV, et al. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest 1976;58:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowe JW, Young JB, Minaker KL, et al. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes 1981;30:219–25. [DOI] [PubMed] [Google Scholar]