Abstract
Objective: To study the possible occurrence of left ventricular (LV) systolic and diastolic asynchrony in patients with systolic heart failure (HF) and narrow QRS complexes.
Design: Prospective study.
Setting: University teaching hospital.
Patients: 200 subjects were studied by echocardiography. 67 patients had HF and narrow QRS complexes (≤ 120 ms), 45 patients had HF and wide QRS complexes (> 120 ms), and 88 served as normal controls.
Interventions: Echocardiography with tissue Doppler imaging was performed using a six basal, six mid-segmental model.
Main outcome measures: Severity and prevalence of systolic and diastolic asynchrony, as assessed by the maximal difference in time to peak myocardial systolic contraction (TS) and early diastolic relaxation (TE), and the standard deviation of TS (TS-SD) and of TE (TE-SD) of the 12 LV segments.
Results: The mean (SD) maximal difference in TS (controls 53 (23) ms v narrow QRS 107 (54) ms v wide QRS 130 (51) ms, both p < 0.001 v controls) and in TS-SD (controls 17.0 (7.8) ms v narrow QRS 33.8 (16.9) ms v wide QRS 42.0 (16.5) ms, both p < 0.001 v controls) was prolonged in the narrow QRS group compared with normal controls. Similarly, the maximal difference in TE (controls 59 (19) ms v narrow QRS 104 (71) ms v wide QRS 148 (87) ms, both p < 0.001 v controls) and in TE-SD (controls 18.5 (5.8) ms v narrow QRS 33.3 (27.7) ms v wide QRS 48.6 (30.2) ms, both p < 0.001 v controls) was prolonged in the narrow QRS group. The prevalence of systolic and diastolic asynchrony was 51% and 46%, respectively, in the narrow QRS group, and 73% and 69%, respectively, in the wide QRS group. Stepwise multiple regression analysis showed that a low mean myocardial systolic velocity from the six basal LV segments and a large LV end systolic diameter were independent predictors of systolic asynchrony, while a low mean myocardial early diastolic velocity and QRS complex duration were independent predictors of diastolic asynchrony.
Conclusions: LV systolic and diastolic mechanical asynchrony is common in patients with HF with narrow QRS complexes. As QRS complex duration is not a determinant of systolic asynchrony, it implies that assessment of intraventricular synchronicity is probably more important than QRS duration in considering cardiac resynchronisation treatment.
Keywords: echocardiography, heart failure, electrocardiography, asynchrony
Treatment of congestive heart failure (HF) to reduce its morbidity and mortality remains one of the major challenges in health care practice. As HF is a debilitating disease, treatment effectively improves symptoms and hence the quality of life is of paramount importance. Recently in a subset of patients with HF with wide QRS complexes signifying electromechanical delay, cardiac resynchronisation in the form of biventricular pacing was shown to achieve these goals irrespective of the aetiology of HF.1–3 Despite careful patient selection, some did not respond to biventricular pacing based on QRS complex duration.4,5 In addition, baseline6–8 or shortening of QRS complex duration4 did not predict haemodynamic, clinical, or echocardiographic improvement. On the other hand, recent studies observed that the severity of systolic asynchrony is a much better predictor of such a response after biventricular pacing7,8 and is much better than baseline QRS complex duration.4 In addition, systolic synchronicity improved after cardiac resynchronisation.7–9 Therefore, it is likely that surface electrocardiography is not sensitive enough to detect the presence and severity of electromechanical delay resulting in asynchronous contraction. Furthermore, it is not known whether the presence of HF may also result in left ventricular (LV) systolic asynchrony even without apparent electrical delay on the ECG. Also, the occurrence of diastolic asynchrony in these patients has not been explored.
In the non-invasive assessment of LV synchronicity, tissue Doppler imaging has been shown to be useful to quantify regional motion, determine the severity of LV systolic asynchrony, and confirm its improvement after biventricular pacing in patients with HF.7–10 Determination of diastolic function quantitatively is also clinically useful.10–12 Therefore, the present study was conducted to assess whether LV systolic and diastolic asynchrony were present in patients with HF and narrow QRS complexes by comparing them with HF patients with wide QRS complexes and with normal controls. In addition, the potential predictors of severity of systolic and diastolic asynchrony were determined.
METHODS
Patients
One hundred and twelve patients (75% men) who had been admitted to hospital for clinical signs and symptoms of HF and had impaired systolic function by echocardiography (ejection fraction < 50%) were recruited. They were stabilised by anti-HF medications and underwent elective tissue Doppler imaging 4–6 weeks later. Of these, 71 had coronary artery disease, 32 had dilated cardiomyopathy, 6 had hypertensive heart disease, 2 had alcoholic cardiomyopathy, and 1 had aortic valve heart disease. Patients with coronary artery disease had either previous evidence of myocardial infarction (n = 52) diagnosed by standard criteria or angiographic evidence of significant disease with or without previous percutaneous coronary intervention (n = 19). Patients with atrial fibrillation were excluded from the study. Among the participating patients, QRS complexes were narrow (> 120 ms; narrow QRS group) in 67 (60%) and prolonged (< 120 ms; wide QRS group) in 45 (40%) (table 1). The results of echocardiography were compared with those of 88 normal healthy volunteers (62% men, χ2 = 1.31, NS v HF group). The volunteers had no history of cardiovascular or systemic diseases and had normal physical examination, and electrocardiographic and echocardiographic findings.
Table 1.
Clinical characteristics of patients with heart failure with narrow and wide QRS complexes
| Narrow QRS (n=67) | Wide QRS (n=45) | χ2 | P value | |
| Age (years) | 65.7 (13.2) | 67.6 (10.4) | NS | |
| Male:female (%) | 73:27 | 78:22 | 0.31 | NS |
| QRS duration (ms) | 99 (11) | 155 (23) | <0.001 | |
| Causes of heart failure (%) | ||||
| Coronary artery disease | 70 | 54 | 6.23 | NS |
| Dilated cardiomyopathy | 21 | 40 | ||
| Hypertension | 6 | 4 | ||
| Valve disease | 1.5 | 0 | ||
| Alcoholic cardiomyopathy | 1.5 | 2 | ||
| Diabetes mellitus (%) | 33 | 39 | 0.41 | NS |
| Hypertension (%) | 50 | 42 | 0.50 | NS |
| Medications (%) | ||||
| ACEI | 72 | 71 | 0.01 | NS |
| Diuretics | 62 | 83 | 5.82 | 0.02 |
| Aspirin | 69 | 53 | 2.52 | NS |
| β Blockers | 30 | 34 | 0.14 | NS |
| Nitrates | 53 | 44 | 0.90 | NS |
| Calcium antagonists | 9 | 5 | 0.38 | NS |
| Digitalis | 16 | 19 | 0.21 | NS |
| Lipid lowering drugs | 35 | 21 | 2.37 | NS |
ACEI, angiotensin converting enzyme inhibitors.
Echocardiography
Standard echocardiography with Doppler studies were performed (System 5, Vingmed-General Electric, Horten, Norway). LV dimension and ejection fraction were measured by two dimensional guided M mode echocardiography according to the guidelines of the American Society of Echocardiography.13 Tissue Doppler imaging was performed in the apical views (four chamber, two chamber, and long axis) for the long axis motion of the LV as previously described.12,14 In brief, two dimensional echocardiography with tissue Doppler colour imaging was performed with a 2.5 or 3.5 MHz phase array transducer. The system was set by bypassing the high pass filter, while the low frequency Doppler shifts were input directly into an autocorrelator.15 Gain settings, filters, and pulse repetitive frequency were adjusted to optimise colour saturation, and a colour Doppler frame scanning rate of 100–140 Hz was used. At least three consecutive beats were stored and the images were digitised and analysed off line by a computer (EchoPac 6.3, Vingmed-General Electric). Myocardial regional velocity curves were constructed from the digitised images.16 For detail assessment of regional myocardial function, the sampling window was placed at the myocardial segment of interest. In each view, both the basal and mid segments were assessed. In this way, the following segments were interrogated: septal, anteroseptal, anterior, lateral, inferior, and posterior segments at both basal and middle levels. For the measurement of timing, the beginning of the QRS complex was used as the reference point, where the time to peak myocardial sustained systolic (TS) and early diastolic velocities (TE) was quantified.9 For the assessment of synchronicity, the standard deviation of TS (TS-SD) and TE (TE-SD) of all 12 LV segments and the maximal difference in TS and TE between any two of the LV segments were calculated. To assess global cardiac function, the mean myocardial sustained systolic (mean SM) and early diastolic (mean EM) velocities from the six basal segments were calculated.14
Statistical analysis
Data were analysed using a statistical software program (SPSS for Windows, version 10.0.7, SPSS Inc, Chicago, Illinois, USA). For comparison of parametric variables between the three groups, analysis of covariance was used to examine the effect of age and heart rate on the dependent variables, followed by one way analysis of variance with the Scheffé correction for significance. Linear regression analysis was performed to investigate the correlation between parametric variables. Categorical data between two or more groups were compared by the Pearson χ2 test. Stepwise multiple regression analysis was performed to assess potential independent covariates on systolic and diastolic asynchrony. The results are expressed as mean (SD). A probability value of p < 0.05 was considered to be significant.
RESULTS
There was no difference in age between the patient group as a whole and normal controls (66 (12) v 64 (10) years, NS). The heart rate was observed to be faster in the patient group than in controls (73 (15) v 65 (11) beats/min, p < 0.001). However, age and heart rate were not confounders of systolic and diastolic asynchrony according to analysis of covariance, and hence unadjusted p values are presented. Table 1 shows the comparison of baseline clinical characteristics between the narrow and wide QRS groups. The age, sex, and aetiology of HF were not different between the two groups. The prescription of medications was also similar except for a slightly greater use of diuretics in the wide QRS group. In the wide QRS group, 23 had a left bundle branch block pattern, 10 had a right bundle branch block pattern, and 12 had intraventricular conduction delay. The mean LV ejection fraction was lower in the patient group than in controls (37.6 (9.6)% v 78.7 (5.7)%, p < 0.001). The wide QRS group had significantly lower LV ejection fraction (33.2 (10.8)% v 40.6 (7.2)%, p < 0.001) and larger LV end diastolic (6.7 (1.1) v 6.1 (0.9) cm, p = 0.001) and end systolic (5.9 (1.1) v 5.1 (0.9) cm, p < 0.001) diameters than the narrow QRS group.
Systolic asynchrony
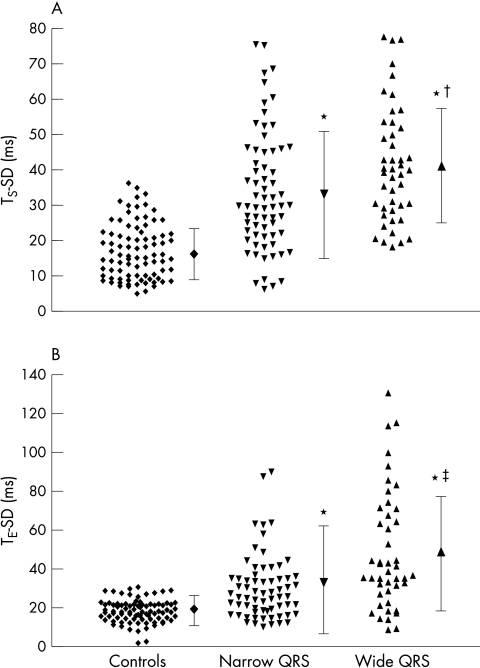
The systolic synchronicity was impaired in patients with HF. The maximal difference in TS was prolonged in the narrow QRS group compared with normal controls (p < 0.001) and was the longest in the wide QRS group (table 2, fig 1). Similarly, the TS-SD was significantly prolonged in the narrow QRS group (p < 0.001), though it was further increased in the wide QRS group (table 2, fig 2A). When a maximal difference of TS of > 100 ms was used to define significant systolic asynchrony, it was not found in the control group but was present in 34 (51%) patients in the narrow QRS group and in 33 (73%) in the wide QRS group (χ2 = 83.2, p < 0.001). When a TS-SD of > 32.6 ms (+ 2 SD of normal controls) was used to define significant systolic asynchrony, it was present in only 3 (3%) control subjects but in 29 (43%) patients in the narrow QRS group and in 29 (64%) in the wide QRS group (χ2 = 58.3, p < 0.001). In addition, systolic asynchrony was more prevalent in patients with wide than in those with narrow QRS complexes by both maximal difference in TS (χ2 = 5.7, p = 0.02) and in TS-SD (χ2 = 4.8, p = 0.03).
Table 2.
Comparison of time to peak myocardial sustained systolic velocity (TS) (in ms) between heart failure patients with narrow and wide QRS complexes and normal controls
| Segment | 1. Controls (n=88) | 2. Narrow QRS (n=67) | 3. Wide QRS (n=45) | p Value (1 v 2) | p Value (1 v 3) | p Value (2 v 3) |
| Basal septal | 127 (19) | 165 (40) | 192 (52) | <0.001 | <0.001 | 0.001 |
| Basal anteroseptal | 124 (18) | 165 (43) | 176 (45) | <0.001 | <0.001 | NS |
| Basal anterior | 125 (26) | 179 (56) | 185 (43) | <0.001 | <0.001 | NS |
| Basal lateral | 131 (23) | 184 (58) | 211 (56) | <0.001 | <0.001 | 0.01 |
| Basal posterior | 131 (25) | 180 (50) | 204 (50) | <0.001 | <0.001 | 0.02 |
| Basal inferior | 129 (23) | 177 (46) | 193 (47) | <0.001 | <0.001 | NS |
| Mid-septal | 130 (28) | 173 (45) | 206 (62) | <0.001 | <0.001 | 0.001 |
| Mid-anteroseptal | 125 (25) | 158 (43) | 183 (63) | <0.001 | <0.001 | 0.02 |
| Mid-anterior | 126 (29) | 177 (55) | 179 (51) | <0.001 | <0.001 | NS |
| Mid-lateral | 125 (29) | 191 (66) | 195 (57) | <0.001 | <0.001 | NS |
| Mid-posterior | 125 (25) | 184 (55) | 201 (60) | <0.001 | <0.001 | NS |
| Mid-inferior | 136 (30) | 195 (59) | 212 (53) | <0.001 | <0.001 | NS |
| Maximal difference in TS | 53 (23) | 107 (54) | 130 (51) | <0.001 | <0.001 | 0.02 |
| TS-SD | 17.0 (7.8) | 33.8 (16.9) | 42.0 (16.5) | <0.001 | <0.001 | 0.009 |
Data are mean (SD).
Maximal difference in TS, maximal difference in time to peak myocardial sustained systolic velocity among all 12 left ventricular segments; TS-SD, standard deviation of the time to peak myocardial sustained systolic velocity of all 12 left ventricular segments.
Figure 1.
Scatterplot showing (A) the distribution of the standard deviation of the time to peak myocardial sustained systolic velocity (TS-SD) and (B) early diastolic velocity (TE-SD) of all 12 left ventricular segments in normal controls, in patients with heart failure and narrow QRS complexes, and in patients with heart failure and wide QRS complexes. *p < 0.001 v controls; †p = 0.009 v normal QRS group; ‡p = 0.002 v normal QRS group.
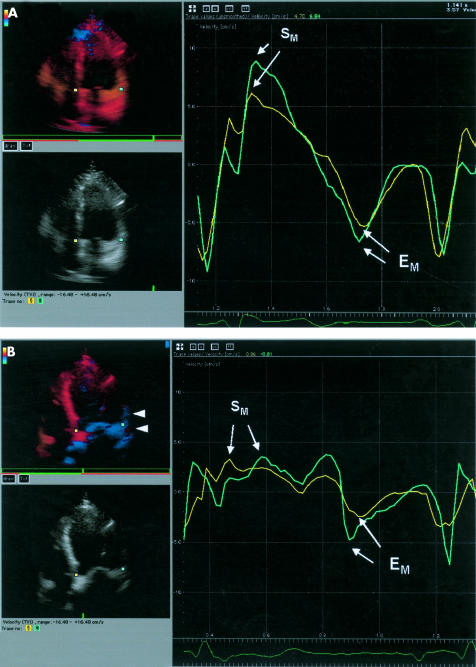
Figure 2.
Regional myocardial velocity curves obtained by tissue Doppler imaging at the basal septal (yellow) and basal lateral (green) segments. In the left hand panels the systolic movement of the myocardium is encoded by colour Doppler with movement towards the probe shown in red and that away from the probe in blue. (A) In the normal control, there is simultaneous peaking of systolic contraction (SM) and early diastolic relaxation (EM) in the basal septal and basal lateral segments in the apical four chamber view. Tissue colour tracking shows systolic contraction in red in both regions. (B) In patients with heart failure and normal QRS duration, there is a dramatic reduction in the amplitude of peak SM and peak EM in both regions. In addition, there is delay in reaching peak SM in the basal lateral segment EM of 120 ms. A difference in peak early diastolic relaxation of 60 ms is also seen. Tissue tracking in early systole shows a delayed systolic movement in the basal and mid-lateral segments, shown in blue (arrow heads).
Table 2 shows the regional TS in individual LV segments. It was significantly delayed in all 12 LV segments in both narrow and wide QRS groups compared with normal controls. In addition, it was further prolonged in some of the LV segments in the wide QRS group compared with the narrow QRS group.
Diastolic asynchrony
Similar to systole, diastolic asynchrony was also evident in patients with HF. The maximal difference in TE was prolonged in the narrow QRS group (p < 0.001) and was the longest in the wide QRS group (table 3, fig 1). In addition, TE-SD was significantly longer in the narrow QRS group than in the normal controls (p < 0.001) and was further prolonged in the wide QRS group (table 3, fig 2B). When a maximal difference of TE of > 100 ms was used to define significant diastolic asynchrony, it was not found in the normal controls but was present in 31 patients (46%) in the narrow QRS group and 31 (69%) in the wide QRS group (χ2 = 71.5, p < 0.001). When a TE-SD of > 30.1 ms (+ 2 SD of normal controls) was used to define significant diastolic asynchrony, it was present in only 2 (2%) of control subjects but in 29 (43%) patients in the narrow QRS group and in 33 (73%) in the wide QRS group (χ2 = 73.0, p < 0.001). In addition, diastolic asynchrony was significantly more prevalent in the wide than in the narrow QRS group by both maximal difference in TE (χ2 = 5.6, p = 0.02) and in TE-SD (χ2 = 9.8, p = 0.002). There was only a modest correlation between systolic and diastolic synchronicity as shown by TS-SD and TE-SD (r = 0.40, p < 0.001). In addition, in patients with narrow QRS complexes, the prevalence of merely abnormal TS-SD was 27%, of merely abnormal TE-SD was 27%, and of abnormal TS-SD and TE-SD was 16%, which was different from those with wide QRS complexes with a prevalence of 16%, 24%, and 49%, respectively (χ2 = 15.5, p = 0.001).
Table 3.
Comparison of time to peak myocardial early diastolic velocity (TE) (in ms) between heart failure patients with narrow and wide QRS complexes and normal controls
| Segment | 1. Controls (n=88) | 2. Narrow QRS (n=67) | 3. Wide QRS (n=45) | p Value (1 v 2) | p Value (1 v 3) | p Value (2 v 3) |
| Basal septal | 515 (39) | 556 (86) | 559 (75) | 0.001 | 0.002 | NS |
| Basal anteroseptal | 532 (42) | 544 (63) | 561 (84) | NS | 0.047 | NS |
| Basal anterior | 526 (41) | 545 (65) | 541 (91) | NS | NS | NS |
| Basal lateral | 519 (40) | 539 (73) | 544 (74) | NS | NS | NS |
| Basal posterior | 519 (41) | 531 (71) | 558 (97) | NS | 0.02 | NS |
| Basal inferior | 514 (41) | 546 (70) | 560 (85) | 0.02 | 0.001 | NS |
| Mid-septal | 534 (38) | 545 (72) | 556 (92) | NS | NS | NS |
| Mid-anteroseptal | 543 (40) | 543 (67) | 553 (78) | NS | NS | NS |
| Mid-anterior | 540 (38) | 545 (71) | 521 (74) | NS | NS | NS |
| Mid-lateral | 538 (41) | 537 (74) | 542 (80) | NS | NS | NS |
| Mid-posterior | 538 (47) | 545 (80) | 547 (100) | NS | NS | NS |
| Mid-inferior | 539 (40) | 568 (70) | 576 (86) | 0.004 | 0.007 | NS |
| Maximal difference in TE | 59 (19) | 104 (71) | 147 (87) | <0.001 | <0.001 | 0.001 |
| TE-SD | 18.5 (5.8) | 33.3 (27.7) | 48.6 (30.2) | <0.001 | <0.001 | 0.002 |
Data are mean (SD).
Maximal difference in TE, maximal difference in time to peak myocardial early diastolic velocity among all 12 left ventricular segments; TE-SD, standard deviation of the time to peak myocardial early diastolic velocity of all 12 left ventricular segments.
Table 3 shows the regional TE in individual LV segments. Unlike TS, TE was delayed in only 3 of 12 LV segments in the narrow QRS group and in 5 of 12 LV segments in the wide QRS group.
Predictors of systolic and diastolic asynchrony
Clinical and echocardiographic predictors of systolic asynchrony were sought. In a univariate model there was no relation between maximal difference in TS or TS-SD and the duration of the QRS complex. However, low LV ejection fraction, mean SM, and mean EM, as well as an enlarged LV significantly correlated with more severe systolic asynchrony (all p < 0.001) (table 4). In the stepwise multiple regression model, only a low mean SM (p = 0.02) and a large LV end systolic diameter (p = 0.009) predicted the severity of systolic asynchrony (table 5).
Table 4.
Correlation between systolic or diastolic asynchrony and various clinical and echocardiographic parameters
| Maximal difference in TS | TS-SD | Maximal difference in TE | TE-SD | |||||
| r | p Value | r | p Value | r | p Value | r | p Value | |
| Age | 0.12 | NS | 0.08 | NS | 0.15 | 0.04 | 0.12 | 0.05 |
| Heart rate | 0.14 | NS | 0.15 | 0.04 | 0.18 | 0.01 | 0.17 | 0.01 |
| QRS duration | 0.11 | NS | 0.12 | NS | 0.35 | <0.001 | 0.35 | <0.001 |
| Ejection fraction | −0.60 | <0.001 | −0.60 | <0.001 | −0.46 | <0.001 | −0.44 | <0.001 |
| Mean SM | −0.60 | <0.001 | −0.60 | <0.001 | −0.39 | <0.001 | −0.37 | <0.001 |
| Mean EM | −0.50 | <0.001 | −0.50 | <0.001 | −0.50 | <0.001 | −0.46 | <0.001 |
| LVDd | 0.55 | <0.001 | 0.55 | <0.001 | 0.46 | <0.001 | 0.42 | <0.001 |
| LVDs | 0.61 | <0.001 | 0.61 | <0.001 | 0.49 | <0.001 | 0.46 | <0.001 |
LVDd, left ventricular end diastolic diameter; LVDs, left ventricular end systolic diameter; Mean EM, mean myocardial early diastolic velocity from the six basal left ventricular segments; Mean SM, mean myocardial sustained systolic velocity from the six basal left ventricular segments.
Table 5.
Stepwise multiple regression analysis comparing the correlation between systolic or diastolic asynchrony and various clinical and echocardiographic parameters
| Maximal difference in TS | TS-SD | Maximal difference in TE | TE-SD | |||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| Age | 0.08 | NS | 0.04 | NS | −0.12 | NS | −0.03 | NS |
| Heart rate | −0.05 | NS | −0.04 | NS | 0.06 | NS | 0.05 | NS |
| QRS duration | −0.15 | NS | −0.13 | NS | 0.71 | <0.001 | 0.25 | <0.001 |
| Ejection fraction | −0.03 | NS | −0.03 | NS | 0.15 | NS | 0.12 | NS |
| Mean SM | −8.32 | 0.02 | −2.67 | 0.02 | 0.19 | NS | 0.18 | NS |
| Mean EM | −0.05 | NS | −0.04 | NS | −13.69 | <0.001 | −4.57 | <0.001 |
| LVDd | −0.001 | NS | −0.03 | NS | 0.09 | NS | 0.07 | NS |
| LVDs | 12.23 | 0.009 | 3.89 | 0.009 | 0.04 | NS | 0.02 | NS |
For diastolic asynchrony, univariate analysis found that all the tested parameters significantly correlated with diastolic asynchrony. However, the stepwise multiple regression model found that only a low mean EM (p < 0.001) and prolonged QRS complex duration (p < 0.001) were independent predictors of diastolic asynchrony (table 5).
DISCUSSION
The present study illustrates the changes in systolic and diastolic synchronicity in patients with HF. Irrespective of QRS duration, patients with HF could develop mechanical asynchrony in both systole and diastole. Although the condition was more prevalent in the wide QRS group, it was not uncommon in patients with narrow QRS complexes. Among various clinical and echocardiographic predictors of asynchrony, it was observed that poor systolic function and enlarged LV predicted systolic asynchrony, while poor diastolic function and prolonged QRS complex duration predicted diastolic asynchrony.
Systolic asynchrony in patients with HF
Systolic asynchrony is characteristic of patients with HF who have a wide QRS complex, which signifies electromechanical delay.4,9,17 In this highly selected group of patients, biventricular pacing has been shown to alleviate symptoms effectively, improve cardiac function,1 and reverse LV remodelling.2,3,9,18 Despite stringent patient selection, some did not respond to biventricular pacing, even though they had wide QRS complexes.4,5,18 Furthermore, neither baseline6–8 nor shortening of QRS complex duration4 were consistent predictors of haemodynamic, clinical, or echocardiographic improvement after biventricular pacing. On the other hand, the mechanism of benefit of biventricular pacing is attributed to the improvement of intraventricular systolic synchrony, which was shown recently by means of tissue Doppler imaging, where the TS-SD was shortened and the intersegmental difference in TS was minimised after biventricular pacing.7,9,10 Also, in patients undergoing biventricular pacing, systolic asynchrony, but not QRS complex duration, proved to be a better predictor of acute haemodynamic,4 echocardiographic,8 or clinical response.7 Despite improving knowledge of systolic synchronicity in HF patients with wide QRS complexes, it remains unclear whether cardiac diseases may result in systolic asynchrony even in patients with narrow QRS complexes. This study illustrates that systolic asynchrony is a common feature in these patients. Two criteria (maximal intersegmental difference in TS of > 100 ms and TS-SD of > 32.6 ms) consistently found that significant systolic asynchrony occurred in > 40% of patients with narrow QRS complexes and at least three quarters of those with wide QRS complexes. Intriguingly, both univariate and multivariate analyses found that the degree of LV asynchrony did not correlate with the duration of QRS complexes. Therefore, electrocardiography is not a good measure of mechanical asynchrony. This may be explained by the following reasons. Firstly, electrocardiography may not be sensitive enough to detect the presence of an electromechanical delay in all the regions of the LV, as it provides only a relatively crude representation of regional electrical conduction. Secondly, some of these patients may have mechanical asynchrony without significant electrical delay in the presence of myocardial disease as a result of deposition of extracellular matrix, myocyte loss, and pathological hypertrophy.19,20 Thirdly, ultrastructural changes within the myocytes secondary to cardiac diseases may adversely affect myocardial contractility, such as a decreased amount of mitochondria and contractile proteins, as well as functional enzymes for oxidative phosphorylation.21 Tissue Doppler imaging has also been used to describe mild systolic asynchrony in patients with LV hypertrophy caused by hypertension, although the QRS duration was not specified in that study.20 Radionuclide ventriculography showed regional systolic asynchrony in patients with coronary artery disease and preserved ejection fraction by using the variation of time to minimal volume.22 More recently, mechanical asynchrony was also described by phase imaging radionuclide ventriculography in four patients with HF and narrow QRS complexes.23 In that study systolic asynchrony was less severe than in those with wide QRS complexes caused by bundle branch block, though no control group was used for comparison.23
Predictors of systolic asynchrony were also sought in the present study. A low mean SM and a large LV end systolic diameter were independent predictors of systolic asynchrony. QRS complex duration was found not to correlate with systolic asynchrony. The mean SM has recently been shown to be a good index of global systolic function and is more sensitive than ejection fraction for detecting LV systolic dysfunction.14 Therefore, it appeared that more severe systolic dysfunction and LV dilatation were associated with more severe systolic asynchrony, independent of QRS complex duration.
Diastolic asynchrony in patients with HF
Similar to systolic asynchrony, diastolic asynchrony also occurred in > 40% of patients with HF and narrow QRS complexes, and in about 70% of those with wide QRS complexes. Although diastolic asynchrony has been described in patients with coronary heart disease and preserved LV function by radionuclide ventriculography,22,24 and recently in patients with LV hypertrophy by tissue Doppler imaging,20 it has not been shown in patients with HF. We observed that diastolic asynchrony occurred as commonly as systolic asynchrony in patients with HF and correlated modestly with QRS complex duration. Therefore, with increasing electromechanical delay resulting in prolongation of QRS duration, diastolic asynchrony worsened. The other predictor of diastolic asynchrony is the degree of diastolic dysfunction, as illustrated by the negative correlation with mean EM. The latter parameter has been reported to be a good index of global diastolic function, which decreased as diastolic dysfunction worsened.14 Interestingly, coexistence of systolic and diastolic asynchrony is more common in patients with wide QRS complexes than in those with narrow ones (49% v 16%), and the correlation between the two conditions is only modest.
Clinical implication
LV systolic and diastolic asynchrony resulted in ineffective contraction and relaxation, respectively. As cardiac output is dependent not only on systolic emptying but also on diastolic filling, systolic and diastolic asynchrony may cause additive haemodynamic compromise in the failing heart. Cardiac resynchronisation has proved to be effective in improving symptoms and systolic function and in reducing LV size in patients with wide QRS complexes,1,7–9,25 as a result of improved LV systolic synchronicity.7–9 However, accurate selection of those who will respond to the treatment is vital and will help to ensure that the treatment is cost effective. This has not been satisfactorily achieved on the basis of the current guidelines, where QRS complex duration is the only surrogate determinant of cardiac asynchrony.1,2,5,26 In patients with wide QRS complexes, systolic asynchrony assessed by magnetic resonance4 or tissue Doppler imaging7,8 was superior to QRS complex duration to predict acute haemodynamic, clinical, or echocardiographic responses. Therefore, direct assessment of systolic synchronicity is probably a better guide for patient selection than the duration of QRS complexes. On the basis of the results of our study, it is imperative to study the efficacy of biventricular pacing in HF patients with narrow QRS complexes who have coexisting mechanical asynchrony. A recent a pilot study reported that biventricular pacing improved functional status of patients with HF with normal QRS duration who had echocardiographic evidence of LV systolic asynchrony.27 However, this result needs to be confirmed by clinical trials with more patients and a longer follow up period.
In conclusion, LV systolic and diastolic asynchrony is not uncommon in patients with systolic HF with normal QRS duration, although it is less prevalent than in those with wide QRS complexes. Therefore, these patients may potentially benefit from cardiac resynchronisation. Selection for such treatment should also be based on information about cardiac synchronicity.
Abbreviations
EM, myocardial early diastolic velocity
HF, heart failure
LV, left ventricular
SM, myocardial sustained systolic velocity
TE, time to peak early diastolic velocity
TE-SD
standard deviation of TE
TS, time to peak myocardial sustained systolic velocity
TS-SD
standard deviation of TS
REFERENCES
- 1.Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873–80. [DOI] [PubMed] [Google Scholar]
- 2.Gras D, Mabo P, Tang T, et al. Multisite pacing as a supplemental treatment of congestive heart failure: preliminary results of the Medtronic Inc. InSync study. Pacing Clin Electrophysiol 1998;21:2249–55. [DOI] [PubMed] [Google Scholar]
- 3.Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation 1999;99:1567–73. [DOI] [PubMed] [Google Scholar]
- 4.Nelson GS, Curry CW, Wyman BT, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation 2000;101:2703–9. [DOI] [PubMed] [Google Scholar]
- 5.Morris-Thurgood JA, Turner MS, Nightingale AK, et al. Pacing in heart failure: improved ventricular interaction in diastole rather than systolic re-synchronization. Europace 2000;2:271–5. [DOI] [PubMed] [Google Scholar]
- 6.Alonso C, Leclercq C, Victor F, et al. Electrocardiographic predictive factors of long-term clinical improvement with multisite biventricular pacing in advanced heart failure. Am J Cardiol 1999;84:1417–21. [DOI] [PubMed] [Google Scholar]
- 7.Ansalone G, Giannantoni P, Ricci R, et al. Doppler myocardial imaging in patients with heart failure receiving biventricular pacing treatment. Am Heart J 2001;142:881–96. [DOI] [PubMed] [Google Scholar]
- 8.Sogaard P, Kim WY, Jensen HK, et al. Impact of acute biventricular pacing on left ventricular performance and volumes in patients with severe heart failure. a tissue Doppler and three-dimensional echocardiographic study. Cardiology 2001;95:173–82. [DOI] [PubMed] [Google Scholar]
- 9.Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002;105:438–45. [DOI] [PubMed] [Google Scholar]
- 10.Garrigue S, Jais P, Espil G, et al. Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol 2001;88:858–62. [DOI] [PubMed] [Google Scholar]
- 11.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–80. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Wang Q, Lau CP, et al. Reversible impairment of left and right ventricular systolic and diastolic function during short-lasting atrial fibrillation in patients with an implantable atrial defibrillator: a tissue Doppler imaging study. Pacing Clin Electrophysiol 2001;24:979–88. [DOI] [PubMed] [Google Scholar]
- 13.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 14.Yu CM, Lin H, Yang H, et al. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 2002;105:1195–201. [DOI] [PubMed] [Google Scholar]
- 15.Miyatake K, Yamagishi M, Tanaka N, et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J Am Coll Cardiol 1995;25:717–24. [DOI] [PubMed] [Google Scholar]
- 16.Gorcsan J, Strum DP, Mandarino WA, et al. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation 1997;95:2423–33. [DOI] [PubMed] [Google Scholar]
- 17.Curry CW, Nelson GS, Wyman BT, et al. Mechanical dyssynchrony in dilated cardiomyopathy with intraventricular conduction delay as depicted by 3D tagged magnetic resonance imaging. Circulation 2000;101:E2. [DOI] [PubMed] [Google Scholar]
- 18.Stellbrink C, Breithardt OA, Franke A, et al. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol 2001;38:1957–65. [DOI] [PubMed] [Google Scholar]
- 19.Weber KT, Anversa P, Armstrong PW, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol 1992;20:3–16. [DOI] [PubMed] [Google Scholar]
- 20.Pai RG, Gill KS. Amplitudes, durations, and timings of apically directed left ventricular myocardial velocities. II. Systolic and diastolic asynchrony in patients with left ventricular hypertrophy. J Am Soc Echocardiogr 1998;11:112–8. [DOI] [PubMed] [Google Scholar]
- 21.Jarreta D, Orus J, Barrientos A, et al. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res 2000;45:860–5. [DOI] [PubMed] [Google Scholar]
- 22.Perrone-Filardi P, Bacharach SL, Dilsizian V, et al. Effects of regional systolic asynchrony on left ventricular global diastolic function in patients with coronary artery disease. J Am Coll Cardiol 1992;19:739–44. [DOI] [PubMed] [Google Scholar]
- 23.Kerwin WF, Botvinick EH, O’Connell JW, et al. Ventricular contraction abnormalities in dilated cardiomyopathy: effect of biventricular pacing to correct interventricular dyssynchrony. J Am Coll Cardiol 2000;35:1221–7. [DOI] [PubMed] [Google Scholar]
- 24.Bonow RO, Bacharach SL, Green MV, et al. Impaired left ventricular diastolic filling in patients with coronary artery disease: assessment with radionuclide angiography. Circulation 1981;64:315–23. [DOI] [PubMed] [Google Scholar]
- 25.Lau CP, Yu CM, Chau E, et al. Reversal of left ventricular remodeling by synchronous biventricular pacing in heart failure. Pacing Clin Electrophysiol 2000;23:1722–5. [DOI] [PubMed] [Google Scholar]
- 26.Butter C, Auricchio A, Stellbrink C, et al. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001;104:3026–9. [DOI] [PubMed] [Google Scholar]
- 27.Sassara M, Achilli A, Fucili S, et al. Efficacy of cardiac resynchronization in narrow QRS patients. Europace 2001;2(suppl B):B59. [Google Scholar]