Abstract
There are two generally proposed causes of dissection of the aorta: (1) cleavage caused by blood entering the tear; and (2) haemorrhage that dissects the media and tear secondary to the cleavage. Using analysis of pressures and forces, this article shows that, on some occasions, these mechanisms alone cannot be responsible for causing aortic dissection
Keywords: dissection, aorta, smooth muscles, force
This article addresses a possible cause of dissection of the aorta. Let us start with a variation of dissection which is not so common—that is, the dissection that has an entry tear located just beyond the left subclavian artery, but which extends proximally into the ascending aorta (in other words, the dissection cleavage propagates from the tear towards the aortic root).1,2
To simplify matters, let us assume that the body is in the supine position. In other positions of the body the calculation is a bit more complicated but the result is the same.
ANALYSIS OF PRESSURES AND FORCES
Forces acting on the flap proximally from the tear
It is thought that cleavage from blood entering through the tear causes the propagation of dissection towards the aortic root. This is represented in fig 1. Points denoted with “F” are in the false channel and those denoted as “T” are in the true channel. If we compare pressure in the true channel at the tear to pressure at the aortic root it is obvious that pressure in the true channel always gradually rises from the tear to the aortic root (so pressure at T1 is greater than pressure at the tear, pressure at T2 is greater than pressure at T1, and pressure at T3 is greater than at T2).
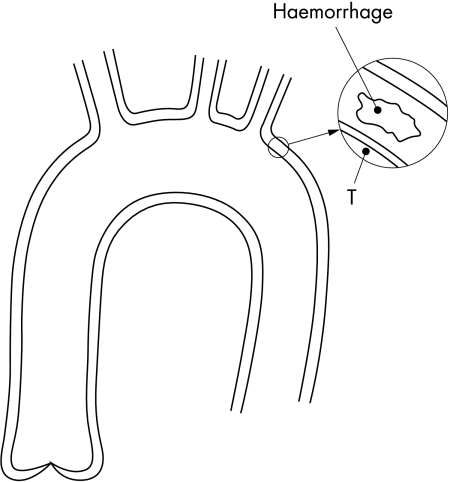
Figure 1.
Calculation of forces that act on the flap. Point F0 is just at the distal end of the dissection.
If there is no flow in the false channel the pressure at any point within the false channel must be the same as the pressure at the tear (so pressure at F0, F1, F2, and F3 is the same as at the tear). When applying these facts to fig 1 it is obvious that pressure at point T1 is greater than the pressure at point F1, that pressure at T2 is greater than pressure at F2, and that pressure at T3 is greater than pressure at F3. This means that the pressure difference pushes the flap towards the adventitia. Therefore, cleavage by blood from the tear cannot be the cause of propagation of dissection from the tear towards the aortic root. This is true no matter how close the tear is to the aortic root. Even if the tear is just above the aortic root (as in type I) it is obvious that the cleavage caused by blood from the tear cannot undermine the aortic valve.
If blood enters from the true channel through the tear into the false channel (that is, flows towards point F3) then pressure at F1 is lower than pressure at the tear (because liquid can flow only from higher pressure towards lower pressure), pressure at F2 is lower than at F1, and pressure at F3 is lower than at F2. Therefore, the pressure difference that pushes the flap towards the adventitia is greater than in the case in which there is no flow in the false channel.
Pressure drop in the aorta
To calculate maximum forces acting on the flap, the maximum pressure difference between a point in the false channel and its most adjacent point in the true channel (for instance, between the points F0 and T0) must be determined. The maximum pressure difference is when there is maximum pressure drop along the aorta. Since the maximum pressure at the aortic root is systolic and minimum pressure at the distal end of the aorta is diastolic, the maximum pressure difference between the aortic root and distal end cannot be greater than systolic pressure minus diastolic pressure. It can also be assumed that pressure linearly falls from the aortic root to the distal end.
So if, for example, systolic pressure is 135 mm Hg, diastolic pressure is 90 mm Hg, and the length of the aorta is 45 cm, then the maximum pressure drop is (135 − 90)/45 = 1 mm Hg/cm (this pressure drop will be used in further calculations).
Forces acting on the flap distally from tear
If the distance between the tear and T0 (fig 1) is 1 cm then pressure at T0 is 1 mm Hg lower than at the tear. When there is no flow in the false channel, the pressure at F0 (point F0 is just at the distal end of the false channel) is equal to the pressure at the tear, so the pressure at F0 is 1 mm Hg higher than at T0. Since the pressure difference at the beginning of the flap (just at the tear) is zero, the mean pressure difference on the flap distally from the tear is (0 + 1)/2 = 0.5 mm Hg. If we suppose, for the purpose of this discussion, that the transverse length of the flap is 5 cm (longitudinal length is equal to distance between tear and F0 or T0—that is, 1 cm) then the area of the flap distal to the tear is 5 × 1 cm2 which, when multiplied by the mean pressure difference on the flap, gives a force by which the flap distal to the tear is pushed towards the true channel.
Converting 0.5 mm Hg to N/m2 gives (1 mmHg = 132.88 N/m2 at 20°C): 0.5 × 132.88 = 66.4 N/m2, and converting centimetres to metres 1 cm = 0.01 m and 5 cm = 0.05 m. Thus, the force is:
 |
This force is too small to cause any further increase of the dissection in the distal direction. If blood enters the false channel then the pressure at F0 is lower than in the case when there is no flow in the false channel, therefore forces on the flap are lower too.
Forces from haemorrhage
It is also thought that haemorrhage dissects the media and that tear is secondary to it. Figure 2 shows a haemorrhage in the media. Point T is the point in the lumen of the aorta which is nearest to the haematoma. For the purpose of this discussion, let us say that the haemorrhage is situated just beyond the left subclavian artery, about 15 cm from the aortic root. If we use the same pressure drop as in the previous example, then pressure at point T is 15 cm × 1 mm Hg/cm = 15 mm Hg lower than at the aortic root. The maximum possible pressure in the haematoma is pressure at the aortic root. This means that pressure in the haematoma cannot be more than 15 mm Hg higher than at point T and from that the maximum force produced by the pressure difference between the haematoma and point T can be calculated.
Figure 2.
Calculation of forces that act from the haemorrhage in the media
Let us suppose that the haematoma has an area of 2 × 2 mm. Converting 15 mm Hg to N/m2 gives: 15 × 132.88 = 1993 N/m2, and converting millimetres to metres, 2 mm = 0.002 m. Thus, the maximum force that is produced by the pressure difference between the haematoma and T is:
 |
That force is too small to make any cleavage in the media or to tear the intima. If there is haemorrhage at the aortic root then the pressure difference between the haematoma and the most adjacent point to it in the lumen of the aorta is zero; therefore it is obvious that there is no force at all and that blood from that haematoma cannot undermine the aortic valve.
THE REAL CAUSE OF DISSECTION
Formation of the dissection
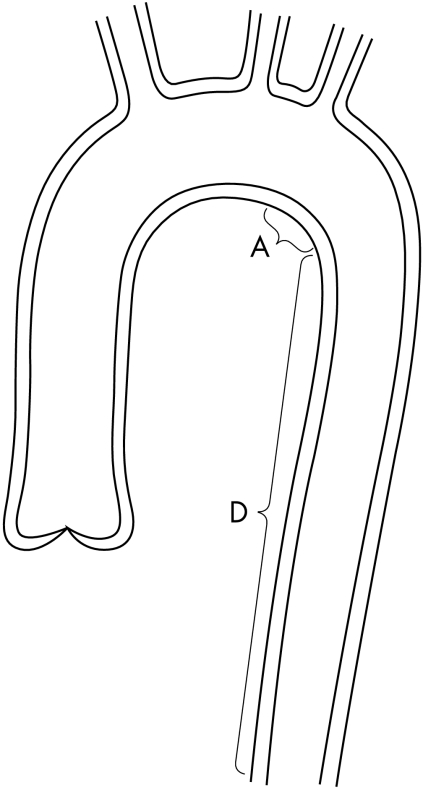
If the aortic smooth muscles contract the adventitia will follow that contraction. But as seen in fig 3, at the area of the aorta where the left subclavian, left carotid, and brachiocephalic artery originate, downward movement of the aortic wall is very limited by those arteries. Upward movement of area A of the aorta is very limited by area D of the aorta. So at the segment of the aorta which comprises the origin of the left subclavian artery and area A, the aortic wall is fixed at the upper and the lower part, so at the upper and lower part of the segment the adventitia can only partially follow the contraction of smooth muscles (fig 4). As a consequence high stresses develop in the media in that segment. If too strong a contraction of smooth muscles (spasm) occurs, dissection of the media will take place, especially if there are areas of cystic degeneration in the media and/or if there is any other weakness in the media.
Figure 3.
Limitation of upward movement of area A by area D.
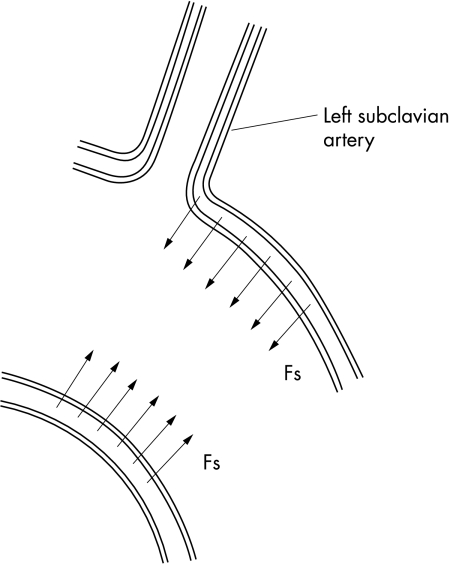
Figure 4.
Forces Fs from smooth muscles cause stresses in the media.
Why does dissection take place just beyond the origin of the left subclavian artery and not at area A? It is because the smooth muscles of the aorta (and thus also forces from the smooth muscles) are not arranged around the origin of the left subclavian artery as evenly as they are at area A. This uneven distribution causes the concentration of forces in the aortic media at the origin of the left subclavian artery which then causes dissection.
Eventually, if the forces from the smooth muscles continue to increase, the intima and part of the media on the smooth muscle will break and a tear will result.
Propagation of dissection
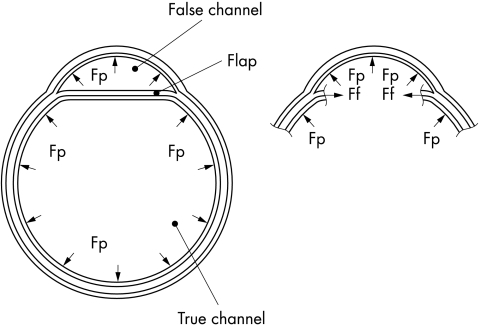
Figure 5 shows a transverse cross section of dissection through the flap. It can be seen that blood pressure forces act on all the inside surfaces of the false and true channels. Assuming, for simplicity’s sake, that pressures both in the true and in the false channel are the same, the forces acting on the flap from the false channel and from the true channel are also equal (and so cancel each other) and therefore they are not shown.
Figure 5.
Transverse (perpendicular to the long axis of the aorta) cross section of a dissection. The left and right cross sections are identical, but in the right cross section the flap is not present so that forces Ff can be shown. Forces from blood pressure acting on the flap from the false channel and the true channel are equal and cancel each other, and therefore they are not shown. Fp, forces from blood pressure in the aorta; Ff, forces with which the flap pulls the aortic wall.
The forces shown stretch the aortic wall. While stretching, the aortic wall stretches the flap in the direction that is perpendicular to the long axis of the aorta and parallel with the flap. Because each force must have a counter force of the same intensity and opposite direction it means that the flap pulls the aortic wall by force Ff (fig 5, right). That force is caused by elasticity of the flap and/or the presence of forces from smooth muscles in the flap. This force Ff by which the flap pulls the aortic wall tends to damage the media further and to increase dissection in the transverse direction. The increasing dissection in the transverse direction is accompanied by movement of the flap towards the centre of the aorta. This movement pulls the rest of the flap which is located distally and/or proximally from the part of the flap shown in cross section, and that causes further dissection in the distal and/or proximal direction.
If blood pressure rises the stretching of the flap increases; consequently the forces that tend to damage the media and to increase dissection also rise. Once the flap, which is perpendicular to the long axis of the aorta, reaches a certain length (the length depending on pressure in the aorta), the elastic forces from the flap alone are sufficient to increase dissection further.
If a tear does not occur, blood from haemorrhage could slowly fill the false channel, thus establishing in the false channel the same pressure as in the aorta, as occurs when there is a tear. But, because of low inflow of blood from haemorrhage in this case, dissection can propagate only very slowly.
CONCLUSION
Dissection takes place as a consequence of major contraction of the smooth muscles, especially when there are areas of cystic degeneration in the media, and haemorrhage takes place because of dissection of the media. Administration of β blockers and/or calcium antagonists will reduce this strong contraction of the smooth muscles, thus reducing dissection.3 On the other hand, discontinuation of long term therapy with β blockers may provoke dissection.4
Fibrous tissue at the aortic root also fixes the aortic wall, which makes this part of the aorta also suitable for initiating dissection. This fixing of the aortic wall explains why more than 95% of dissections begin at two places: either just above the aortic valve or just beyond the origin of the left subclavian artery.5
In hypertensives there is an increase in smooth muscle mass and that also produces a stronger force.6,7 That, together with acquired cystic degeneration in hypertensives, may explain why dissection takes place mostly in hypertensives.
Smooth muscles in the media in large, elastic arteries are generally helically oriented.8 Therefore it is readily possible that dissection propagates in a direction perpendicular to the orientation of these helically oriented smooth muscles (analogous to “normal” propagation which is perpendicular to circumferentially oriented smooth muscles). As a result spiral dissection develops, known as “spiral barber pole” dissection.1
Supplementary Material
REFERENCES
- 1.Schlant RC, Alexander RW. Hurst’s the heart, 8th edn. New York: McGraw-Hill, 1994:2170.
- 2.Hurst JW, Alpert JS. Diagnostic atlas of the heart. New York: Raven Press, 1994:337.
- 3.Hoshino T, Ohmae M, Sakai A. Spontaneous resolution of a dissection of the descending aorta after medical treatment with a beta blocker and a calcium antagonist. Br Heart J 1987;58:82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eber B, Tscheliessnigg KH, Anelli-Monti M, et al. Aortic dissection due to discontinuation of beta-blocker therapy. Cardiology 1993;83:128–31. [DOI] [PubMed] [Google Scholar]
- 5.Hurst JW, Alpert JS. Diagnostic atlas of the heart. New York: Raven Press, 1994:338.
- 6.Owens GK, Schwartz SM. Alterations in vascular smooth muscle mass in the spontanteneously hypertensive rat. Role in cellular hypertrophy, hyperploidy and hyperplasia. Circ Res 1982;51:280–9. [DOI] [PubMed] [Google Scholar]
- 7.Owens GK, Schwartz SM. Vascular smooth muscle cell hypertrophy and hyperploidy in the Goldblatt hypertensive rat. Circ Res 1983;53:491–501. [DOI] [PubMed] [Google Scholar]
- 8.Schlant RC, Alexander RW. Hurst’s the heart, 8th ed. New York: McGraw-Hill, 1994:31.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.