Increased expression of tumour necrosis factor α (TNFα) has been found in cardiac tissue of patients with chronic heart failure, and the failing heart has been suggested as the cause of immune activation.1
Another, less frequently reported facet of the macrophage derived mediator TNFα is its function as an angiogenic factor2 which may be induced by myocardial ischaemia. This pleiotropic cytokine appears to act also as an angioinhibitory factor depending on the concentration, location, and duration of its action.3 So far, there have been no studies in patients with coronary artery disease (CAD) investigating the relation between the potentially angiogenic TNFα and directly measured collateral flow, the latter representing a variable in the course of CAD with considerable impact on its outcome.
The purpose of this study was to test the hypothesis that there is an association between TNFα and collateral flow in patients with CAD and normal systolic ventricular function.
METHODS
Patients
One hundred patients (mean (SD) age 65 (11) years, 70 men, 30 women) with one vessel and two vessel CAD were included in the study. All underwent percutaneous transluminal coronary angioplasty (PTCA) of one stenotic lesion because of symptoms related to stable CAD. Patients were prospectively selected on the basis of the following criteria: (1) no previous infarction in the myocardial area undergoing PTCA; (2) normal left ventricular ejection fraction; (3) no congestive heart failure; (4) no baseline ECG ST segment abnormalities; (5) no clinical or laboratory signs of inflammatory illness; (6) absence of overt neoplastic disease; and (7) no chronic obstructive pulmonary disease. The study population was divided into two groups according to the presence (insufficient collaterals, n = 78) or absence (sufficient collaterals, n = 22) of intracoronary ECG signs of myocardial ischaemia during the first one minute balloon occlusion of the stenosis to be revascularised.
The present investigation was approved by the institutional ethics committee, and the patients gave informed consent to participate in the study.
Coronary collateral assessment
In all study patients, coronary collateral flow relative to normal antegrade flow through the non-occluded coronary artery (collateral flow index (CFI)) was determined using coronary pressure measurements. A 0.014 inch pressure monitoring PTCA guidewire (Pressure Wave, Jomed, Switzerland) was set at zero, calibrated, advanced through the guiding catheter, and positioned distal to the stenosis to be dilated. CFI was determined by simultaneous measurement of mean aortic pressure (Pao, mm Hg, via the angioplasty guiding catheter), the distal coronary artery pressure during balloon occlusion (Poccl, mm Hg), and the central venous pressure (CVP, mm Hg, via a right atrial catheter). CFI was calculated as:
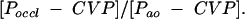 |
Determination of TNFα
The blood samples obtained simultaneously with the CFI measurement from the ostium of the vessel (10 ml) were collected in sterile tubes (anticoagulant: sodium citrate), placed on ice, treated by centrifugation at 3000 g for 15 minutes at 4°C, and the plasma was frozen at −70°C. Plasma concentrations of TNFα were measured by an enzyme linked immunosorbent assay with a monoclonal antibody specific for TNFα (Biosource International, Camarillo, California, USA). The sensitivity of the test is equivalent to TNFα > 1 pg/ml.
RESULTS
Patient characteristics and clinical data
There were no significant differences between the two study groups regarding age of the patients, sex, body mass index, heart rate, and mean blood pressure before PTCA, or left ventricular ejection fraction and filling pressure. Also, there were no significant differences in the frequency of cardiovascular risk factors, recent non-Q wave myocardial infarctions, and the use of aspirin or vasoactive drugs.
TNFα concentration and coronary collateral flow data
Several variables for the assessment of the collateral circulation were significantly different among the groups (table 1).
Table 1.
Coronary angiographic and collateral data, TNFα
| Insufficient collaterals | Sufficient collaterals | p Value | |
| Number of patients | 78 | 22 | |
| Coronary angiography | |||
| One/two vessel disease | 59%/41% | 62%/38% | NS |
| Number of vessels diseased | 1.7 (0.7) | 1.8 (0.8) | NS |
| Vessel (PTCA): LAD/LCX/RCA | 55%/15%/30% | 23%/68%/9% | 0.06 |
| Diameter stenosis (%) | 75 (15) | 87 (11) | 0.001 |
| Collateral assessment | |||
| Angiographic degree (before occlusion, 0–3) | 0.4 (0.7) | 1.3 (1.1) | 0.0001 |
| Angina pectoris during PTCA | 6 (7%) | 6 (27%) | 0.001 |
| Collateral flow index (no unit) | 0.17 (0.12) | 0.34 (0.15) | 0.0001 |
| TNFα detectable (> 1 pg/ml) | 41 (53%) | 6 (27%) | 0.03 |
| TNFα (pg/ml) | 30.3 (79.7) | 1.9 (6.5) | <0.05 |
Data presented as mean (SD) unless otherwise indicated.
LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; NS, not significant; PTCA, percutaneous transluminal coronary angioplasty; RCA, right coronary artery; TNFα, tumour necrosis factor α.
TNFα values were found at detectable plasma concentrations (> 1 pg/ml) in 47% of all patients. Detectable TNFα concentrations were measured significantly more often in patients with insufficient collaterals than in those with sufficient collaterals, and TNFα concentrations were higher in the former group of patients than the latter (table 1).
There was a significant, inverse correlation between plasma TNFα concentrations and CFI values; this was the case for the entire study group (CFI = 0.23–0.004 TNFα; r = −0.21, p = 0.04) as well as for patients having detectable (> 1pg/ml) TNFα concentrations (CFI = 0.25–0.001 TNFα; r = −0.35, p = 0.02).
DISCUSSION
This study in patients with CAD and normal systolic left ventricular function documents that serum TNFα was detectable in almost half the individuals investigated, and that TNFα was inversely related to simultaneously measured coronary collateral flow.
TNFα in patients without congestive heart failure
In 1996, Torre-Amione and colleagues demonstrated that the non-failing human heart does not express TNFα, whereas the end stage failing human heart re-expresses large amounts of protein.1 Conversely, our study in patients with CAD and stable angina pectoris, but with entirely normal systolic function, found detectable TNFα concentrations in 47% of patients.
TNFα as angiogenic or angioinhibitory factor
The present work provides several independent lines of evidence for the existence of an inverse relation between TNFα and collateral flow: (a) using a dichotomous, ECG based characterisation of coronary collaterals, the detectability of TNFα occurs in approximately half the patients with insufficient collaterals and in only one fourth of the individuals with collaterals sufficient to prevent myocardial ischaemia during vessel occlusion; (b) continuous TNFα values in all patients are also significantly different between the two collateral groups; and (c) using continuous measurements for both variables, TNFα and collateral flow, there is an inverse association irrespective of whether all or just the detectable values (> 1 pg/ml) are analysed. Our study was not designed to elucidate whether this inverse relation between intracoronary TNFα and directly and simultaneously measured collateral flow is causal, whether it reflects an angiogenic action of the cytokine with consequent feedback suppression of TNFα production, whether TNFα elicits angioinhibitory properties on the coronary circulation, or whether TNFα is upregulated very swiftly in patients with poorly developed collaterals in response to myocardial ischaemia even during a coronary occlusion as brief as one minute.
TNFα has been documented to induce capillary vessel formation in the rat cornea and the chick chorioallantoic membrane at low doses.2 In vitro, TNFα has been shown to stimulate chemotaxis of bovine capillary endothelial cells and to induce these cells to form capillary tube-like structures.2 Considering the above mentioned experimental work, it can be speculated that the inverse relation between TNFα and collateral flow index found in the present study reflects a situation whereby augmented expression of TNFα is needed in patients with poorly grown collaterals, and it is downregulated in patients with sufficient collaterals.
In vitro data documenting angioinhibitory properties of TNFα would allow an opposite interpretation of our data to the one outlined above5: the predominantly elevated TNFα concentrations in patients with low collateral flow reflect the ongoing inhibitory action of the cytokine on collateral development.
De novo production of TNFα in response to the very brief ischaemic stimulus used in our study is an unlikely explanation for the raised TNFα concentrations in patients with collaterals insufficient to prevent ischaemia. This is because blood samples for TNFα determination were collected simultaneously with, and not after, the collateral flow measurements during which the occlusion occurred.
Acknowledgments
Supported by a grant from the Swiss National Science Foundation, grant 32-58945.99
Abbreviations
CAD, coronary artery disease
CFI, collateral flow index
CVP, central venous pressure
Pao, mean aortic pressure
Poccl, coronary artery pressure during balloon occlusion
PTCA, percutaneous transluminal coronary angioplasty
TNFα, tumour necrosis factor α
REFERENCES
- 1.Torre-Amione G, Kapadia S, Benedict C, et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the studies of left ventricular dysfunction (SOLVD). J Am Coll Cardiol 1996;27:1201–6. [DOI] [PubMed] [Google Scholar]
- 2.Leibovich SJ, Polverini PJ, Shepard HM, et al. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 1987;329:630–2. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida A, Ono M, Shono T, et al. Involvement of interleukin-8, vascular endothelial growth factor and basic fibroblast growth factor in tumor necrosis factor-alpha- dependent angiogenesis. Molecular and Cellular Biology 1996;17:4015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiler C, Fleisch M, Garachemani AR, et al. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol 1998;32:1272–9. [DOI] [PubMed] [Google Scholar]
- 5.Frater-Schröder M, Risau W, Hallmann R, et al. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci 1987;84:5277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


