Abstract
Purpose: To compare percutaneous coronary intervention (PCI) using stent implantation versus coronary artery bypass graft (CABG) in patients with multiple vessel disease with involvement of the proximal left anterior descending coronary artery (LAD).
Methods: 230 patients with multiple vessel disease and severe stenosis of the proximal LAD (113 with PCI, 117 with CABG). They were a cohort of patients from the randomised ERACI (Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease) II study.
Results: Both groups had similar baseline characteristics. There were no significant differences in 30 day major adverse cardiac events (death, myocardial infarction, stroke, and repeat procedures) between the strategies (PCI 2.7% v CABG 7.6%, p = 0.18). There were no significant differences in survival (PCI 96.4% v CABG 95%, p = 0.98) and survival with freedom from myocardial infarction (PCI 92% v CABG 89%, p = 0.94) at 41.5 (6) months’ follow up. However, freedom from new revascularisation procedures (CABG 96.6% v PCI 73%, p = 0.0002) and frequency of angina (CABG 9.4% v PCI 22%, p = 0.025) were superior in the CABG group.
Conclusion: Patients with multivessel disease and significant disease of the proximal LAD randomly assigned in the ERACI II trial to PCI or CABG had similar survival and survival with freedom from myocardial infarction at long term follow up. Repeat revascularisation procedures were higher in the PCI group.
Keywords: coronary artery bypass, proximal LAD intervention, multivessel coronary stenting, multivessel revascularisation
Several randomised trials comparing balloon angioplasty (percutaneous transluminal coronary angioplasty (PTCA)) versus coronary artery bypass graft (CABG) in patients with multivessel coronary artery disease have shown no significant differences in mortality and in the incidence of acute myocardial infarction between these treatment strategies. CABG has a prognosis advantage over PTCA only in insulin dependent and orally treated diabetic patients.1–9
Data from non-randomised studies suggest that the outcome after revascularisation depends on the distribution of prerevascularisation coronary artery disease.10,11 In that regard, the severity and location of left anterior descending coronary artery (LAD) involvement have been identified as important determinants of outcome in patients with coronary artery disease. Several registries of PTCA versus CABG have shown a trend to improved survival with CABG in patients with multiple vessel disease and proximal LAD stenosis.10–15 However, a major limitation of these studies is that they were conducted in the era preceding the widespread use of coronary stenting. We recently published the results of the ERACI (Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease) II trial, a randomised comparison of percutaneous coronary intervention (PCI) with liberal use of coronary stents versus CABG in patients with multiple vessel disease.16 The purpose of the present study was to analyse the immediate and long term outcome of PCI versus CABG in a cohort of patients with multiple vessel disease and significant proximal LAD disease randomised in the ERACI II trial.
MATERIALS AND METHODS
Study population
From 450 patients in the ERACI II study we identified 414 patients with severe stenosis of the LAD; 230 of them had severe (≥ 70%) stenosis in the proximal LAD (from its origin to the takeoff of the first diagonal branch). They constituted the study population. Patients with severe stenosis of the LAD distal to the first diagonal branch were excluded from this analysis.
Details of the ERACI II trial have been previously described.16 It is of particular importance that > 90% of the patients randomised in this study presented with unstable angina. The primary end point of the study was to compare major adverse cardiac events defined as death, Q wave myocardial infarction, stroke, and repeat PTCA or CABG at 30 days and at one, three, and five years of follow up. Death included mortality from all causes. Secondary end points were follow up angina status, comparison of the completeness of revascularisation as assessed by stress thallium, and follow up cost of the two revascularisation strategies.
Revascularisation techniques
CABG procedure was performed by standard surgical techniques. Complete revascularisation was achieved, when possible, by using arterial conduits and reverse saphenous vein grafts.
Coronary angioplasty and stent deployment were performed using standard techniques as previously described.16–18 Weight based intravenous heparin was given to achieve an activated clotting time > 280 seconds during the procedure. Patients with rest pain in the preceding 48 hours and those with postmyocardial infarction angina received a bolus followed by an infusion of abciximab. Only vessels with a reference diameter > 3 mm by visual estimation were stented electively. Target vessels were stented with the Gianturco Roubin II stent (Cook Inc, Bloomington, Indiana, USA) as the primary device.
Complete anatomical revascularisation was defined as the angiographic absence of a residual stenosis ≥ 70% in any major epicardial vessel after PCI. In the case of CABG it was determined by the number of distal anastomoses in diseased vessels previously identified as target arteries during the planned surgical strategy. Complete functional revascularisation was determined by stress thallium performed in the first month after the initial revascularisation strategy.7
Statistical analysis
Angiographic and clinical outcomes were analysed according to intention to treat. Results are expressed as mean (SD). Continuous variables of the two treatment groups were compared by unpaired two tailed Student’s t test. Categorical variables and the 30 day composite end point of the two groups were compared by the χ2 method. The composite clinical end point during the follow up period was compared by the Kaplan-Meier and Wilcoxon tests with p values calculated according to the log rank test.19 All tests were two tailed, and p < 0.05 was considered to indicate significance.
RESULTS
Patient population and baseline characteristics
From 230 patients in the ERACI II trial with ostial or proximal LAD stenosis, 113 were randomly assigned to undergo PCI and 117 to CABG. After randomisation eight patients in the CABG group did not receive the index procedure and seven of them crossed over to the PCI group. In contrast, two patients randomly assigned to undergo PCI crossed over to the CABG group (6.8% v 1.7%, respectively, p = 0.138).
There were no significant differences in age, sex, and the frequency of current smokers, diabetes, hypercholesterolaemia, unstable angina class IIb, IIIb, and C, and previous myocardial infarction between the two groups of patients (table 1). Angiographic characteristics of both groups of patients were also similar, exhibiting a large proportion of two vessels disease (64% in CABG and 62% in PCI, NS).
Table 1.
Baseline demographic, clinical, and angiographic characteristics of patients with LAD ostial or proximal lesion in the ERACI II study
| PCI (n=113) | CABG (n=117) | p Value | |
| Men (%) | 77 | 77 | NS |
| Women (%) | 23 | 23 | NS |
| Age >65 years (%) | 50 | 38.5 | NS |
| Hypertension (%) | 68.5 | 66 | NS |
| Smokers (%) | 48 | 43.5 | NS |
| Diabetes (%) | 15.9 | 19 | NS |
| High cholesterol (%) | 63 | 57 | NS |
| Previous infarction (%) | 17.5 | 20.5 | NS |
| Obesity (%) | 15.3 | 19 | NS |
| Peripheral vascular disease (%) | 16 | 15 | NS |
| Unstable angina, class II, III, C (%) | 91 | 90.5 | NS |
| Two vessel disease (%) | 62 | 64 | NS |
| Three vessel disease (%) | 38 | 36 | NS |
CABG, coronary artery bypass graft surgery; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention.
Revascularisation procedures
Patients in the CABG group each received an average of 2.4 distal anastomoses. A left internal mammary graft to the LAD was used in 93% of the CABG patients. In the PCI group an average of 1.7 stents per patient were used, and 28.3% of PCI patients received a bolus followed by an infusion of abciximab during the procedure.
Results of coronary angioplasty
Successful revascularisation—defined as a successful dilatation of at least one major epicardial vessel (residual stenosis ≤ 30%) without occurrence of death, Q wave myocardial infarction, emergent hospital CABG, or repeat emergent PCI—was achieved in 97.4% of the PCI patients. At least one vessel was successfully treated in all PCI patients. Two vessels were successfully treated in 84.3% of the patients. The planned PCI strategy was successfully accomplished in 93.6% of intended coronary vessels.
Complete anatomical (CABG 81% v PCI 77%, p = 0.862) and functional (CABG 90.2% v PCI 88%, p = 0.995) revascularisation was similar with both revascularisation strategies. Similar functional revascularisation was apparent by comparable normal, reversible, and non-reversible thallium perfusion defect areas in the PCI and CABG groups at 30 day follow up thallium stress test.
In-hospital and 30 day outcomes
There were no significant differences in 30 day mortality (CABG 2.5% v PCI 0%, NS) between the groups of patients. There were three (2.5%) deaths in the CABG group: two patients died during the initial hospitalisation and one patient died within 30 days after randomisation but before the index procedure could be performed.
The 30 day incidence of Q wave myocardial infarction was similar in both groups (PCI 1.8% v CABG 5.1%, NS). The 30 day composite end point of death and myocardial infarction was 1.8% v 7.6% for the PCI and CABG groups, respectively (p = 0.089). While two patients in the PCI group needed an emergent PTCA (one of them suffered a myocardial infarction), no patient in the CABG group required an emergent repeat revascularisation procedure. No patient in either group suffered a periprocedural major stroke. The 30 day composite end point of major adverse cardiac events (death, myocardial infarction, repeat revascularisation procedure, and stroke) was similar in both groups of patients (2.7% v 7.6% for PCI and CABG, respectively, p = 0.18) (table 2).
Table 2.
In-hospital and 30 day results in patients with LAD ostial or proximal lesion in the ERACI II study
| PCI (n=113) | CABG (n=117) | p Value | |
| Death (%) | 0 | 2.5 | NS |
| Q wave MI (%) | 1.8 | 5.1 | NS |
| Death + MI (%) | 1.8 | 7.6 | 0.089 |
| Emergent PTCA (%) | 1.8 | 0 | NS |
| Emergent CABG (%) | 0 | 0 | NS |
| Repeat PTCA (%) | 1.8 | 0 | NS |
| MACE (%) | 2.7 | 7.6 | NS |
MACE, major adverse cardiac events (death + myocardial infarction (MI) + stroke + repeat PTCA or CABG).
Late clinical follow up
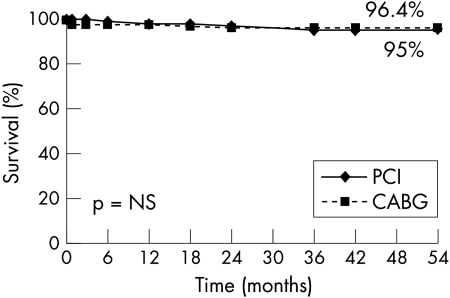
Patients were followed up clinically for a mean of 41.5 (6) months (range 31–54 months). Clinical follow up data were available for 228 (99.1%) patients. Kaplan-Meier analysis showed similar two year survival (PCI 96.4% v CABG 95%, p = 0.98) with both strategies of revascularisation (fig 1). After hospital discharge, a similar number of patients died in each group (four in the PCI group and three in the CABG group).
Figure 1.
Kaplan-Meier curve showing survival at follow up of patients with ostial or proximal left anterior descending (LAD) coronary artery randomly assigned to undergo percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG).
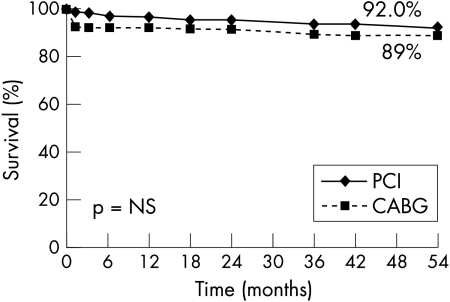
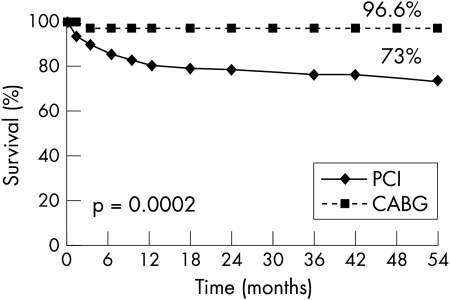
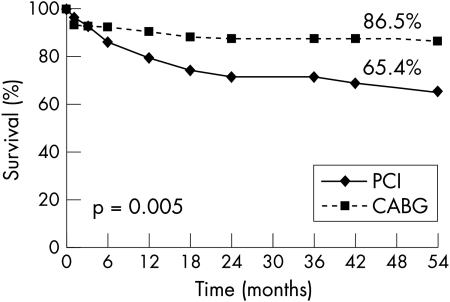
Similarly, Kaplan-Meier curves showed comparable survival with freedom from non-fatal myocardial infarction (PCI 92% v CABG 89%, p = 0.94) in both groups of patients (fig 2). In contrast, fig 3 shows freedom from new revascularisation procedures was significantly lower with CABG (96.6% v 73%, p = 0.0002). As a consequence solely of this greater need for repeat revascularisation procedures with PCI, Kaplan-Meier event-free survival (freedom from death, myocardial infarction, and repeat PTCA or CABG) was better with CABG than with PCI (86.5% v 65%, p = 0.005) (fig 4).
Figure 2.
Kaplan-Meier curve showing freedom from death and myocardial infarction of patients with ostial or proximal LAD randomly assigned to undergo PCI or CABG.
Figure 3.
Kaplan-Meier curve showing freedom from repeat PCI or CABG of patients with ostial or proximal LAD randomly assigned to undergo PCI or CABG.
Figure 4.
Kaplan-Meier curve showing freedom from major adverse cardiac events (death, myocardial infarction, stroke, and repeat PCI or CABG) of patients with ostial or proximal LAD randomly assigned to undergo PCI or CABG.
Angina occurred more frequently during follow up in the PCI group (22% v 9.4%, p = 0.025). Nevertheless, by the end of the follow up period a similar number of patients in each group were either asymptomatic or had angina class I symptoms (PCI 98% v CABG 96.4%, NS). Furthermore, only 6.2% of patients in the PCI group crossed over to the CABG group during follow up.
Diabetic patients treated with PCI (n = 18) or CABG (n = 22) had similar long term survival (PCI 100% v CABG 95.4%). However, there was a trend for greater target vessel revascularisation (33.3% v 13.6%, p = 0.27) in diabetic patients treated with PCI.
Hospital and follow up costs
Since analysis was performed by intention to treat, group charges included those of the patients that crossed over to the other revascularisation strategy. In Argentina, at the time of the ERACI II study, the average cost was US$4500 for PCI (excluding stents) and $11000 for uncomplicated CABG. These costs included hospital charges (two days for PCI and nine days for CABG), fees, and an honorarium for both procedures. The costs of stents ranged from $2500–$3000. Each additional day in the coronary care unit added a cost of $600–$1100. The use of abciximab in bolus form and 12 hours infusion added a cost of $3000.
With the above numbers, the final 30 day cost was $1 124 300 for the PCI group versus $1 230 500 for the CABG group (p = 0.73). During follow up, requirement for additional revascularisation procedures added a cost of $283 000 for the PCI group and $32 000 for the CABG group. There were no significant differences in overall cost per patient for the entire follow up period (PCI $12 472 v CABG $10 790, p = 0.18).
DISCUSSION
The present study suggests that patients with multiple vessel disease and severe stenosis of the proximal LAD can be effectively treated with either PCI with liberal use of stents or CABG. Although the number of patients in this subset analysis of the ERACI II trial is small, at long term follow up both revascularisation strategies had similar survival, survival with freedom from non-fatal myocardial infarction, and completeness of revascularisation. Thus, both strategies seem equally safe and effective in improving the clinical status of these patients.
In addition, our study shows that even in the current PCI era characterised by high stent utilisation, requirements for repeat revascularisation procedures remain significantly higher with percutaneous interventions that with conventional bypass surgery. However, they are lower than previously reported with the use of conventional balloon angioplasty.1–9 Although the incidence of angina during the entire follow up was more frequent with stents, it decreased over time and by the end of follow up angina class was similar in both groups. Furthermore, in our study only 6% of PCI patients crossed over to surgery during three years of follow up, a value significantly lower than the > 20% previously reported in non-stent series.1–9
Finally, the three year follow up costs for both procedures were similar, suggesting that, compared with CABG, stenting is no longer cost effective. An increase of resource costs for PCI techniques using stents as compared with plain balloon angioplasty used previously in the ERACI I trial is responsible for this finding.10
Comparison with previous studies
Several registries have previously reported that CABG had better survival than PTCA when the proximal segment of the LAD was involved, and suggested that CABG should be the first treatment option in these cases.10–15 However, a major limitation of these studies is that they were conducted in the era preceding the widespread use of coronary stenting. Moreover, there are no data from randomised trials to support this recommendation. Furthermore, the presence of LAD stenosis was not identified as a factor of poor survival after PTCA in the seven year BARI (bypass angioplasty revascularization investigation) follow up study.9
We recently published the 30 day and one year follow up outcomes of the total cohort of patients randomised in the ERACI II trial.16 In-hospital results of the ERACI II trial showed a higher incidence of major adverse events in the surgical group. In contrast, in the present study of patients with multivessel disease and significant proximal LAD stenosis, in-hospital and 30 day surgical mortality and major adverse cardiac events were similar in both groups of patients and were lower than the rates previously reported for the overall population of the ERACI II trial.16 As we previously suggested for the ERACI II trial, baseline patient characteristics more likely account for these results.16 Our cohort of patients with multivessel coronary artery disease and proximal LAD stenosis had similar age, angina status, and presence of diabetes than the overall ERACI II trial group. However, they had a lower incidence of significant triple vessel disease (36% v 62%, p = 0.008) and a lower incidence of associated peripheral vascular disease (15% v 27%, p = 0.042) than the overall cohort of ERACI II patients. Diffuse coronary artery disease and concomitant peripheral vascular disease are well recognised comorbidities associated with an increased hospital surgical risk.20–23 Moreover, the results of the present study are in agreement with those of the ARTS (artery revascularization strategy study) trial showing no significant differences in mortality between the two modalities.24 In fact our hypothesis is supported by the similar angiographic characteristics of the ARTS and the cohort of patients of the ERACI II trial with proximal LAD stenosis (33% v 36% incidence of triple vessel disease, respectively). Our results and those of the ARTS trial are in contrast with those of the SOS (stent or surgery) trial showing an increased mortality in the stent arm.25
Role of coronary stenting
The use of stents during coronary angioplasty has been shown to reduce acute complications during the initial procedure.26 The liberal use of stents in this study explains why emergent CABG and acute closure were significantly lower than previously reported with PTCA before the stent era.1–9 Stents are also associated with a lower incidence of clinical and angiographic restenosis.27–29 Although a liberal use of stents was adopted in the present study, the requirement for repeat revascularisation procedures and the incidence of angina were significantly higher in the PCI than in the CABG group. Nevertheless, revascularisation procedures in the PCI group of the present study are lower than we previously reported in the ERACI I trial at the same follow up period with the use of conventional balloon angioplasty.1–7 Similar results have been reported by the ARTS and SOS investigators.24,25
The use of a left internal mammary graft to the LAD is associated with better outcome than with saphenous vein graft.30,31 In agreement with the present study, a small randomised study comparing mammary artery graft versus stenting in patients with single LAD stenosis had similar safety results but a lower incidence of angina and requirement for repeat procedures with surgery.32
Although in the present study the number of repeat revascularisation procedures was greater in the PCI group, both treatment strategies achieved similar survival, freedom from myocardial infarction, and completeness of revascularisation. Interestingly, after hospital discharge, survival curves over three years of follow up were parallel, providing further support that the only limitation of stent implantation compared with surgery is restenosis. Since stenting and PTCA are less invasive and less traumatic than surgery, but equally safe, it is reasonable to recommend PCI for the treatment of patients with multiple vessel coronary artery disease with involvement of the proximal LAD.
Limitations of the study
This study was a retrospective analysis of a randomised subpopulation of the ERACI II trial. It is a post hoc analysis of a subgroup of patients with proximal LAD stenosis, which was not predefined in the original ERACI II trial. The number of patients is small with only 280 patients eligible for this subset analysis. In this trial the Gianturco Roubin II stent was used. This coil design stent has been shown to have a higher restenosis rate than slotted tube stents.33 Thus, the incidence of new revascularisation procedures may have been lower had a tubular stent design been used instead. Finally, this study was performed in the pre-eluting stent era. Applying the results from eluting stent studies to patients with multiple vessel disease should decrease the need for repeat revascularisation procedures, which is the only disadvantage of PCI compared with CABG.34
Acknowledgments
This study was supported by an unrestricted grant from Cook, Inc (Bloomington, Indiana, USA).
Abbreviations
ARTS, artery revascularization strategy study
BARI, bypass angioplasty revascularization investigation
CABG, coronary artery bypass graft
ERACI, Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease
LAD, left anterior descending coronary artery
PCI, percutaneous coronary intervention
PTCA, percutaneous transluminal coronary angioplasty, SOS, stent or surgery
APPENDIX: STUDY ORGANISATION AND PARTICIPANTS
Steering Committee: Alfredo Rodriguez MD PhD FACC, William O’Neill MD FACC, Igor Palacios MD FACC, Liliana Grinfeld MD FACC, José Navia MD FACC, Raul Oliveri MD, Néstor Pérez Baliño MD FACC, Julio Baldi MD.
Safety Committee: Marcelo Elizari MD FACC, Jorge Lerman MD FACC.
Coordinating centre: CECI: Alfredo Rodriguez MD PhD FACC, Víctor Bernardi MD, Sandra Saavedra MD, Máximo Rodríguez Alemparte MD, Cecilia Espinosa BS.
Core laboratory: Carlos Fernández Pereira MD, Omar Santaera MD.
Statistics: Ulises Questa MD PhD, Larry Harrell BS.
Clinical Events Committee: Raúl Oliveri MD, Néstor Pérez Baliño MD FACC, Eduardo Mele MD FACC, Marcelo Garrido MD, Daniel Vogel MD.
Writing Committee: Alfredo Rodriguez MD PhD FACC, José Navia MD FACC, Igor Palacios MD FACC, William O’Neill MD FACC.
Participating centres:Sanatorio Otamendi: Carlos Mauvecin MD, Miguel Russo Felsen MD, Jorge Caviglia MD, Ruben Dayan MD; Sanatorio Anchorena: Diego Guastavino MD, Ernesto Peyregne MD; Hospital Español: Alberto Cristino MD, Fernando Boullon MD; Clinica Provincial: Omar Santaera MD, Luis Pringles MD; Clinica Belgrano: Omar Santaera MD, Alejandro Delacasa MD, Marcelo Martínez Peralta MD; Hospital Italiano: Liliana Grinfeld MD, Daniel Berrocal MD, Jose Gabay MD, Felix Fabrykant MD, Roberto Grinfeld MD; Clinica Olivos: Antonio Pocovi MD, Alberto Domenech MD.
REFERENCES
- 1.Rodríguez A, Boullon F, Perez Baliño N, et al. Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery by pass surgery in multivessel disease (ERACI): in-hospital results and 1 year follow up. J Am Coll Cardiol 1993;33:1060–7. [DOI] [PubMed] [Google Scholar]
- 2.King SB III, Lembo NJ, Weintraub, et al. A randomized trial comparing coronary angioplasty with coronary bypass surgery. Emory angioplasty versus surgery trial (EAST). N Engl J Med 1994;331:1044–50. [DOI] [PubMed] [Google Scholar]
- 3.RITA trial participants. Coronary angioplasty versus coronary artery bypass surgery: the randomized intervention treatment of angina (RITA) trial. Lancet 1993;341:573–80. [PubMed] [Google Scholar]
- 4.CABRI trial participants. Coronary angioplasty vs. bypass revascularization investigation (cabri) results during the first year. Lancet 1995;346:1179–83. [PubMed] [Google Scholar]
- 5.Hamm CW, Reimers J, Ischinger T, et al. A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary artery disease. N Engl J Med 1994;331:1037–43. [DOI] [PubMed] [Google Scholar]
- 6.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in-patients with multivessel disease. N Engl. J Med 1996; 335: 217–25. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez A, Mele E, Peyregne E, et al. Three year follow up the Argentine randomized trial of percutaneous transluminal coronary angioplasty versus coronary artery bypass surgery in multivessel disease (ERACI). J Am Coll Cardiol 1996;27:1178–84. [DOI] [PubMed] [Google Scholar]
- 8.Pocock S, Henderson R, Rickards A, et al. Meta-analysis of randomized trials comparing coronary angioplasty with bypass surgery. Lancet 1995;346:1184–9 [DOI] [PubMed] [Google Scholar]
- 9.The BARI Investigators. Seven-year out come in the bypass angioplasty revascularization investigation (BARI) by treatment and diabetic status. J Am Coll Cardiol 2000;35:1122–9. [DOI] [PubMed] [Google Scholar]
- 10.Hannan EL, Racz MJ, McCallister BD, et al. A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1999;33:63–72. [DOI] [PubMed] [Google Scholar]
- 11.Jones RH, Kesler K, Phillips HR, et al. Long term survival benefits of coronary artery bypass grafting and percutaneous transluminal angioplasty in patients with coronary artery disease. J Thorac Cardiovasc Surg 1996;111:1013–25. [DOI] [PubMed] [Google Scholar]
- 12.Favaloro RG. Critical analysis of coronary artery bypass graft surgery: a 30-year journey. J Am Coll Cardiol 1998;31(suppl B):1B–63B. [DOI] [PubMed] [Google Scholar]
- 13.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease: initial results from the era of coronary angioplasty. Circulation 1994;89:2015–25. [DOI] [PubMed] [Google Scholar]
- 14.Ellis S, Fisher L, Dustman-Ellis S, et al. Comparison of coronary angioplasty with medical treatment for single- and double-vessel coronary disease with left anterior descending coronary involvement: long term outcome based on Emory-CASS registry study. Am Heart J 1989;118:208–19. [DOI] [PubMed] [Google Scholar]
- 15.Arvinder S, Kurbaan AF, Rickards CDJ, et al. Relation between coronary artery disease, baseline clinical variables, revascularization mode, and mortality. Am J Cardiol 2000,86:938–42. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez A, Bernardi V, Navia J, et al. Argentina randomized study : coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple-vessel disease (ERACI II):30 day and one-year follow-up results. J Am Coll Cardiol 2001;37:51–8. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez A, Ayala F, Bernardi V, et al. Optimal coronary balloon angioplasty with provisional stenting versus primary stent (OCBAS): immediate and long-term follow up results. J Am Coll Cardiol 1998;32:1351–7. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez A, Bernardi V, Fernandez M, et al. In-hospital and late results of coronary stents versus conventional balloon angioplasty in acute myocardial infarction (GRAMI trial). Am J Cardiol 1998;81:1286–91. [DOI] [PubMed] [Google Scholar]
- 19.Selvin S. Statistical analysis of Epidemiology data. New York: Oxford University Press, 1991.
- 20.Eagle K, Guyton R. ACC/AHA guidelines for coronary artery bypass graft surgery: a report of the American College of Cardiology American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 1999;34:1262–347. [DOI] [PubMed] [Google Scholar]
- 21.Burek K, Sutto-Tyrrell K, Brooks M, et al. Prognostic importance of lower extremity arterial disease in patients undergoing coronary revascularization in the bypass angioplasty revascularization investigation (BARI). J Am Coll Cardiol 1999;34:716–21. [DOI] [PubMed] [Google Scholar]
- 22.Magovern JA, Sakert T, Magovern GJ, et al. A model that predicts morbidity and mortality after coronary bypass graft surgery. J Am Coll Cardiol 1996;28:1147–53. [DOI] [PubMed] [Google Scholar]
- 23.Jones RH, Hannan E, Hammermeister KE, et al. Identification of preoperative variables needed for risk adjustment of short-term mortality after coronary artery bypass graft surgery. J Am Coll Cardiol 1996;28:1478–87. [DOI] [PubMed] [Google Scholar]
- 24.Serruys PW, Unger F, Sousa E, et al. Comparison of coronary-artery bypass surgery and stenting for the treatment of multivessel disease. N Engl J Med 2001;344:1117–24. [DOI] [PubMed] [Google Scholar]
- 25.Stables R. SOS results. Presented at the Annual Meeting of the American College of Cardiology, 2001, Orlando, Florida.
- 26.Roubin GS, Cannon AD, Agrawal SK, et al. Intracoronary stenting for acute threatened closure complicating percutaneous transluminal coronary angioplasty. Circulation 1992;85:916–27. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW, de Jaegere P, Kiemeneij F, et al, on behalf of the BENESTENT Study Group: A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 1994;331:489–95. [DOI] [PubMed] [Google Scholar]
- 28.Discman DL, Leon MB, Baum DS, et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med 1994;331:426–501. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez A, Santaera O, Larribau M, et al. Coronary stenting decrease restenosis in lesions with early loss in luminal diameter 24hours after successful PTCA. Circulation 1995;91:1397–402. [DOI] [PubMed] [Google Scholar]
- 30.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Hennesey TG, Codd MB, Donnelly S, et al. Long-term outcome following coronary artery bypass grafting for isolated stenosis of the left anterior descending coronary artery. Eur Heart J 1998;19:447–57. [DOI] [PubMed] [Google Scholar]
- 32.Goy JJ, Kaufmann U, Goy Eggenberg D, et al. A prospective randomized trial comparing stenting to internal mammary artery grafting for proximal, isolated de novo left anterior coronary artery stenosis: the SIMA trial. Mayo Clin Proc 2000;75:1116–23. [DOI] [PubMed] [Google Scholar]
- 33.Lansky AJ, Roubin GS, O Shaughnessy CD, et al, for the GR-II Randomized Clinical Trial Investigators. Randomized comparison of GR II stent and Palmaz-Schatz for elective treatment of coronary stenosis. Circulation 2000;102:1364–8. [DOI] [PubMed] [Google Scholar]
- 34.Sousa EJ, Costa M, Abizaid A, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation 2001;103:192–5. [DOI] [PubMed] [Google Scholar]