Growth factors play an important role in the pathogenesis of cardiovascular diseases by regulating cellular proliferation, migration, differentiation, and apoptosis. Vascular endothelial growth factor (VEGF) is a potent mitogen for endothelial cells, and has been reported to promote collateral formation in ischaemic cardiac muscle and tissue repair after wounding.1 In animal models, transfer of the VEGF gene into ischaemic limbs induces angiogenesis and improves tissue perfusion.2 Recently, Baumgartner and colleagues3 demonstrated that intramuscular injection of naked plasmid DNA encoding VEGF improved limb ischaemia by promoting angiogenesis in patients with critical peripheral arterial disease. Pearlman and colleagues1 demonstrated that direct infusion of VEGF improved global left ventricular ejection fraction and regional wall motion after acute myocardial infarction in an animal model. VEGF also induces endothelium dependent relaxation in isolated canine coronary arteries and decreases arterial pressure in rats in vivo. We previously reported that elevation of VEGF concentrations in patients with acute myocardial infarction contributed to the improvement of left ventricular function.4 In the present study, we measured circulating VEGF concentrations in patients with congestive heart failure (CHF).
METHODS
We studied 41 patients (24 men and 17 women, mean (SEM) age 67.4 (1.8) years, range 34–86 years) admitted to Jichi Medical School Hospital for acute exacerbation of CHF (table 1). The diagnosis of heart failure was confirmed in all patients by clinical findings and non-invasive assessment of myocardial function. Left ventricular ejection fraction (LVEF) was determined by echocardiographic evaluation. The control group consisted of 12 healthy volunteers (7 men and 5 women, aged 62.7 (4.1) years, range 34–80 years) without cardiovascular disease. All patients and volunteers gave their written informed consent to participation in the study, and the protocol was approved by the ethics committee of Jichi Medical School.
Table 1.
Characteristics of patients with congestive heart failure
| Male/female (n) | 24/17 |
| Age (years) | 67.4 (1.8) |
| LVEF (%) | 39.0 (2.7) |
| BNP (pg/ml) | 308 (45.1) |
| Underlying heart disease (n) | |
| Ischaemic heart disease | 14 |
| Valvar heart disease | 12 |
| Cardiomyopathy | 7 |
| Arrhythmia | 7 |
| Congenital heart disease | 1 |
| Drugs (n) | |
| Nitrates | 16 |
| ACE inhibitors | 10 |
| β Blockers | 1 |
| Digoxin | 13 |
| Diuretics | 29 |
ACE, angiotensin converting enzyme; BNP, brain natriuretic peptide; CHF, congestive heart failure; LVEF, left ventricular ejection fraction.
We previously found that VEGF concentrations in plasma isolated using heparin as an anticoagulant were underestimated (10.0 (2.5) %) compared to those in serum isolated without heparin.4 Thus, blood samples were obtained without anticoagulants. Blood sampling was performed at admission (on day 1), and on days 7 and 14 after admission using a 21 gauge needle inserted into a large antecubital vein of the patients in the supine position. Serum was obtained by centrifugation and stored immediately at −80°C until assayed for VEGF. VEGF concentrations were determined using a specific enzyme linked immunosorbent assay kit (Amersham International, Buckinghamshire, UK). The standard curve was linear from 15.6 to 1000 pg/ml of VEGF. Concentrations of brain natriuretic peptide (BNP) were determined by radioimmunoassay using a specific anti-BNP antibody (SRL Inc, Tokyo, Japan). The sensitivity of BNP assay was 2.0 pg/ml.
Data are expressed as mean (SEM). Differences were analysed by the non-parametric Mann-Whitney U test. The correlation between two parameters was assessed by the non-parametric Spearman rank correlation test. A probability of p < 0.05 was considered significant.
RESULTS
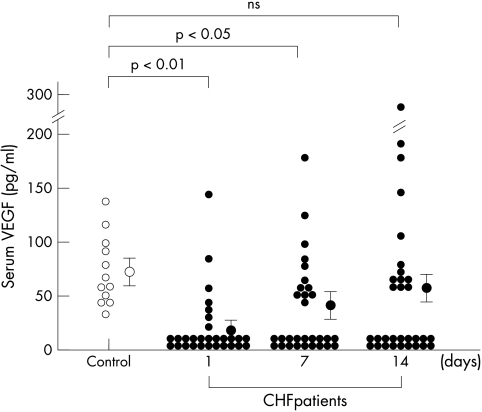
Figure 1 shows the time course of changes in serum VEGF concentrations in patients with CHF after admission. Serum VEGF concentrations in CHF patients at admission (mean 10.3 (4.4) pg/ml; median < 15.6 pg/ml) were significantly lower than those of control subjects (mean 68.7 (16.4) pg/ml; median 63.0 pg/ml) (p < 0.01). Serum VEGF concentrations in CHF patients went up after admission, and the concentrations on day 14 were not significantly different compared with those of control subjects. No correlations were observed between serum VEGF concentrations and LVEF (r = 0.13, p = 0.40) or BNP concentrations (r = −0.22, p = 0.17) at admission.
Figure 1.
Changes in serum VEGF concentrations in patients with CHF. Serum was obtained from peripheral blood without using anticoagulants. The data on day 1 represent serum VEGF concentrations at admission. Serum VEGF concentrations in CHF patients (solid circles) at admission were significantly lower than those in control subjects (open circles). Values are expressed as mean (SEM). NS, not significant.
DISCUSSION
This study revealed a significant decrease in serum VEGF concentration in CHF patients compared with control subjects. The VEGF concentration at admission was not related to the severity of CHF (LVEF and BNP concentrations), and the mechanism by which circulating VEGF concentrations are decreased in CHF is unknown. It may be due to decreased VEGF production and/or enhanced VEGF clearance. Previously, Kraus and colleagues5 reported that VEGF concentrations in skeletal muscle were reduced in patients with CHF. Abraham and colleagues6 revealed the downregulation of VEGF mRNA and protein in the hearts of patients with end stage dilative cardiomyopathy. Cheng and colleagues7 also reported that VEGF concentrations in the pleural fluid of patients with CHF were significantly reduced. These findings suggest that decreased circulating VEGF concentrations in CHF patients are caused by decreased VEGF production in the cardiovascular tissue. On the other hand, circulating VEGF binds to Flt-1 and KDR receptors for VEGF, which are expressed predominantly in vascular endothelial cells,8 and the clearance of circulating VEGF through these receptors may also be related in part to decreased serum VEGF concentrations in CHF patients.
In conclusion, decreased serum VEGF concentrations were found in CHF patients. However, the underlying heart disease of CHF patients was heterogenous in this study and the precise mechanism responsible for the decreased serum VEGF concentrations is still unknown. Further studies are needed to clarify the relation between VEGF and CHF.
Abbreviations
BNP, brain natriuretic peptide
CHF, congestive heart failure, LVEF, left ventricular ejection fraction
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Pearlman JD, Hibberd MG, Chuang ML, et al. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nature Med 1995;1:1085–9. [DOI] [PubMed] [Google Scholar]
- 2.Shimpo M, Ikeda U, Maeda Y, et al. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc Res 2002;53:993–1001. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998;97:1114–23. [DOI] [PubMed] [Google Scholar]
- 4.Hojo Y, Ikeda U, Zhu Y, et al. Expression of vascular endothelial growth factor in patients with acute myocardial infarction. J Am Coll Cardiol 2000;35:968–73. [DOI] [PubMed] [Google Scholar]
- 5.Kraus WE, Duscha BE, Thompson MA, et al. Vascular endothelial growth factor levels in skeletal muscle are reduced in patients with congestive heart failure. Circulation 1999;100:I–246. [Google Scholar]
- 6.Abraham D, Hofbauer R, Schäfer R, et al. Selective downregulation of VEGF-A165, VEGF-R1, and decreased capillary density in patients with dilative but not ischemic cardiomopathy. Circ Res 2000;87:644–7. [DOI] [PubMed] [Google Scholar]
- 7.Cheng D, Rodriguez RM, Perkett EA, et al. Vascular endothelial growth factor in pleural fluid. Chest 1999;116:760–5. [DOI] [PubMed] [Google Scholar]
- 8.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9–22. [PubMed] [Google Scholar]