Abstract
Objective: To investigate the relation between the wall motion abnormalities and sodium channel abnormalities in cases of the Brugada syndrome.
Design: Consecutive prospective case–control study in a single hospital.
Setting: Tertiary referral centre.
Patients: 13 consecutive patients with Brugada syndrome and 13 age and sex matched control subjects.
Interventions: Each subject underwent electron beam computed tomography (EBT) and a 12 lead ECG before and after disopyramide injection.
Main outcome measures: QRS width and the magnitude of ST segment elevation in the 12 lead ECG; wall motion by EBT.
Results: After disopyramide, EBT revealed deterioration of focal wall motion abnormalities in the right ventricular outflow tract region in eight of the 13 patients (62%). Prolongation of the QRS width after disopyramide injection in lead V2, which usually reflects the electrical activity in right ventricular outflow tract region, was greater in these eight patients (p < 0.01) than in the other five patients, in whom wall motion did not change after disopyramide. The degree of augmentation of ST segment elevation did not differ significantly between the two groups
Conclusions: The deterioration of wall motion abnormalities in the right ventricular outflow tract region after disopyramide suggests the presence of functional abnormalities of the sodium channel. Some patients with Brugada syndrome may have arrhythmogenic substrates with abnormal responses to sodium channel blockers.
Keywords: sodium channel blockers, computed tomography, electrocardiography, Brugada syndrome
The Brugada syndrome is associated with a high risk of sudden death.1,2 Several antiarrhythmic agents (sodium channel blockers) are known to modulate ST segment elevation in patients with this syndrome.2–5 Moreover, Chen and colleagues found mutations in the cardiac sodium channel gene, SCN5A, the first gene to be linked with Brugada syndrome, in three families.6 However, the aetiology and arrhythmogenic substrate in this syndrome remain unknown.
Brugada syndrome is believed to occur in individuals with no apparent heart disease. The diagnosis of a structurally normal heart is based on the clinical features and on the results of routine cardiac imaging (coronary angiography, left and right ventriculography, and echocardiography). However, some studies have revealed wall motion abnormalities of the right ventricle in patients with Brugada syndrome.7–10 The relation between these abnormalities and the cardiac sodium channels remains unknown.
Electron beam computed tomography (EBT) is a relatively new and sensitive imaging technique useful in diagnosing several features of arrhythmogenic right ventricular dysplasia11,12 and Brugada syndrome.8 We used EBT to determine whether a class IA sodium channel blocker modulated wall motion in patients with Brugada syndrome, and to explore the relation between the change in wall motion and changes in the characteristics of the 12 lead ECG after drug treatment.
METHODS
Patients
We used a patient–control design. Thirteen consecutive patients with Brugada syndrome admitted to our hospital between December 1998 and December 1999 and 13 controls were studied. The patients were eligible for inclusion in the study if they met all the following criteria:
a history of syncope, cardiac arrest, or aborted sudden cardiac death with or without documentation of ventricular fibrillation
no QT prolongation (corrected QT interval < 440 ms½)
ST segment elevation (coved or saddleback type) in the precordial leads (V1 to V3) with or without right bundle branch block on the 12 lead ECG during sinus rhythm
normal findings on physical examination
no major abnormal findings of either right or left ventricular morphology or function demonstrated by chest radiography, echocardiography, and ventriculography
no significant stenosis detected on coronary angiography.
All 13 patients were men. They ranged in age from 27–62 years (mean (SD), 46 (10) years) (table 1). None of the patients appear in our previous report.8
Table 1.
Clinical characteristics of patients with the Brugada syndrome
| ECG findings during sinus rhythm | Electrophysiology | |||||||
| Patient number | Age (years) | Documented VF | QRS in lead V2 (ms) | Qtc (ms½) | RBBB | ST elevation (max, mm) | HV interval (ms) | Induced VF (pacing site) |
| Deteriorated group | ||||||||
| 1 | 48 | + | 112 | 378 | ICRBBB | V1–3 (7) | 48 | + (RVOT) |
| 2 | 33 | + | 132 | 376 | CRBBB | V1,2 (1) | 52 | + (RVOT) |
| 3 | 39 | − | 96 | 370 | – | V1–3 (4) | 34 | − |
| 4 | 57 | + | 116 | 422 | ICRBBB | V1,2 (1) | 38 | + (RVOT) |
| 5 | 62 | + | 96 | 408 | – | V1–3 (1) | 35 | + (RVOT) |
| 6 | 49 | + | 100 | 380 | ICRBBB | V1,2 (1) | 35 | + (RVOT) |
| 7 | 42 | + | 100 | 376 | ICRBBB | V1,2 (1.6) | 42 | + (RVOT) |
| 8 | 56 | + | 108 | 411 | ICRBBB | V1–3 (2) | 40 | + (RV apex) |
| Unchanged group | ||||||||
| 9 | 51 | + | 88 | 380 | – | V1,2 (1.2) | 32 | + (RVOT) |
| 10 | 43 | + | 108 | 373 | ICRBBB | V1,2 (2.5) | 42 | + (RVOT) |
| 11 | 51 | + | 100 | 420 | ICRBBB | V1,2 (4) | 38 | + (RVOT) |
| 12 | 36 | + | 104 | 368 | ICRBBB | V1–3 (3) | 40 | − |
| 13 | 27 | + | 100 | 370 | ICRBBB | V1–3 (2.8) | 40 | + (RVOT) |
CRBBB, complete right bundle branch block; HV, bundle of His to ventricle; ICRBBB, incomplete right bundle branch block; max, maximum; QTc, corrected QT interval; RBBB, right bundle branch block; RV, right ventricular; RVOT, right ventricular outflow tract; VF, ventricular fibrillation.
The control population consisted of eight age and sex matched healthy volunteers with normal hearts and normal ECGs, and five age and sex matched volunteers with normal hearts but with right bundle branch block, for assessment of the wall motion. Controls ranged in age from 27–57 years (mean 45 (13) years). All controls had normal findings on physical examination and no abnormalities of right or left ventricular morphology or function on chest radiography and echocardiography.
Written informed consent was obtained from all patients and controls, and all underwent EBT and drug provocation testing, as well as an electrophysiological study.
Drug provocation testing
We examined the effects on the 12 lead ECG of a single dose of the class IA sodium channel blocker disopyramide (2 mg/kg), which was infused over 10 minutes in all patients and controls. The 12 lead ECG was recorded at double amplitude (2 cm/mV) and paper speed (50 mm/s) before and three minutes after the completion of the disopyramide infusion. The QRS onset and offset were determined visually in all 12 leads. The magnitude of the ST segment was measured visually at 20 ms after the end of the QRS in only lead V2 because we found that the maximum ST segment elevation occurred in this lead in all patients. We evaluated the change in the ST segment elevation in lead V2 and in the QRS width in all 12 leads.
CT scanning technique
Each subject underwent EBT with a C-150 scanner (Imatron, San Francisco, California, USA). We were able to obtain a near short axis oblique view of the heart with this instrument. Initially, we performed serial volume mode scanning at end systole as previously reported.8 A total dose of 60 ml of non-ionic contrast medium (iopamidol, 370 mg I/ml; Nippon Schering, Osaka, Japan) was injected through the right antecubital vein with a mechanical injector. Using the multislice mode, eight-level scans were obtained with the injection of contrast medium to evaluate the function of both ventricles before and three minutes after completion of the disopyramide infusion, as previously reported.8
Assessment of morphological abnormalities
We evaluated the morphological and wall motion abnormalities visually by EBT. Each study was separately interpreted by two trained cardiologists and a radiologist who were blinded to the patient's clinical status.
We determined the wall thickness and aneurysmal change in both ventricles with volume mode scans, and evaluated any functional abnormalities (wall motion and wall thickening) in each of the chambers with movie mode scans. In the movie mode study, we compared the images before the disopyramide infusion with those obtained three minutes after the completion of the infusion. For visual semiquantitative right ventricular wall motion analysis, we made a 13 segment model of the right ventricle as previously reported.8 For each segment, a score was assigned as follows: normal, 1; hypokinesis, 2; akinesis, 3; dyskinesis, 4. In all studies, focal hypokinesis, akinesis, and dyskinesis were defined, respectively, as decreased systolic excursion, no systolic excursion of a wall segment, and paradoxical motion of a wall segment compared with neighbouring segments, as assessed visually. Deterioration of the wall motion after the disopyramide infusion was defined as a greater decrease or disappearance of the segmental wall motion than before disopyramide. Diffuse deterioration of the wall motion was excepted because it probably represents the negative inotropic effect of disopyramide. Abnormalities were considered to be present if they were observed by two of the three observers. The sum of the wall motion scores for each subject was assessed to determine interobserver and intraobserver variabilities. Intraobserver variability was determined from triplicate measurements. Interobserver variability was determined from measurements by three observers.
We divided the patients into two groups (table 1): a deteriorated group, consisting of patients with deterioration of the wall motion after the disopyramide infusion (n = 8), and an unchanged group, consisting of patients with no deterioration of the wall motion (n = 5). We compared the 12 lead ECG characteristics before and after the disopyramide infusion in these two groups of patients.
Electrophysiological studies
All patients underwent electrophysiological studies in the non-sedated state. All antiarrhythmic drugs were discontinued for at least five drug half lives before the test. A 6 French steerable quadripolar electrode catheter with 2.5 mm interelectrode spacing (EP Technologies Inc, Sunnyvale, California, USA) was introduced percutaneously through the femoral vein and positioned in the right ventricle under fluoroscopic guidance. Bipolar electrograms were recorded at more than 10 sites in the right ventricle. Abnormal bipolar electrograms were considered to be those with a low amplitude (< 1 mV) or long duration (> 70 ms), or both.
Programmed electrical ventricular stimulation was achieved using a 2 ms pulse duration at twice the diastolic threshold, delivered from a programmable stimulator (SEC-3120, Nihon Kohden, Tokyo, Japan). The protocol included an eight beat ventricular paced drive train at two basic cycle lengths (500 and 400 ms), followed by decremental introduction of up to triple extrastimuli at the right ventricular apex and right ventricular outflow tract. The end point was either reproducible induction of ventricular fibrillation or completion of the pacing protocol.
Statistics
Data are presented as mean (SD). Comparison of absolute values of QRS width and ST segment elevation before and after the disopyramide infusion in the two groups was done using repeated measures two way analysis of variance (ANOVA). Comparison of the difference (Δ) in the QRS duration and ST segment elevation before and after the disopyramide infusion was done using one way ANOVA with a multiple comparison test. Assessments of the interobserver and intraobserver variability were also undertaken using the Bland and Altman method. Probability values of p < 0.05 were taken as significant.
RESULTS
The characteristics of the two groups of patients are presented in table 1. None of the patients were related. Their mean age was 48 (10) years in the deteriorated group and 42 (10) years in the unchanged group (NS). Of the patients in the deteriorated group, two had a normal QRS duration, five had incomplete right bundle branch block, and one had complete right bundle branch block. Of the patients in the unchanged group, one had a normal QRS duration and four had incomplete right bundle branch block. All 13 patients had several syncopal episodes with palpitations or aborted sudden cardiac death. Ventricular fibrillation had been documented in 10 of these patients on ECG recordings. The mean age of the controls with or without right bundle branch block was 47 (10) and 43 (12) years, respectively (NS). None of controls had a history of syncopal attacks.
Morphological assessment: assessment of the right ventricle
EBT findings in patients with Brugada syndrome
Before the disopyramide infusion, EBT showed wall motion abnormalities of the right ventricle in nine (69%) of the 13 patients. All these abnormalities were in the anterior basal region of the right ventricular outflow tract (fig 1A). There were no abnormal findings in relation to wall thickness or the focal presence of intramural or transmural fatty tissue.
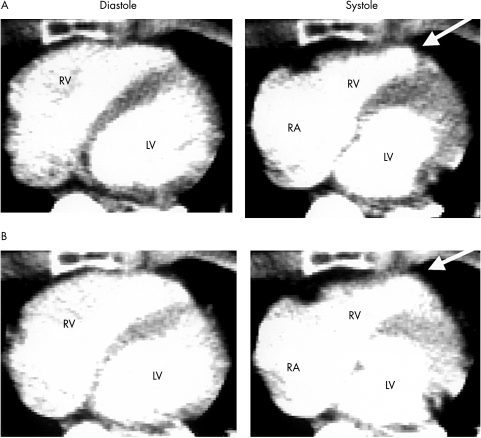
Figure 1.
Representative electron beam computed tomography findings in a patient with Brugada syndrome in the “deteriorated” group (see text for definition of groups). A “near short axis” movie mode scan (acquisition time, 50 ms) was obtained with the administration of contrast medium. (A) Wall motion abnormalities were observed before the disopyramide infusion in the right ventricular outflow tract region (arrowhead). (B) The wall motion abnormalities were impaired after the disopyramide infusion (arrowhead). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
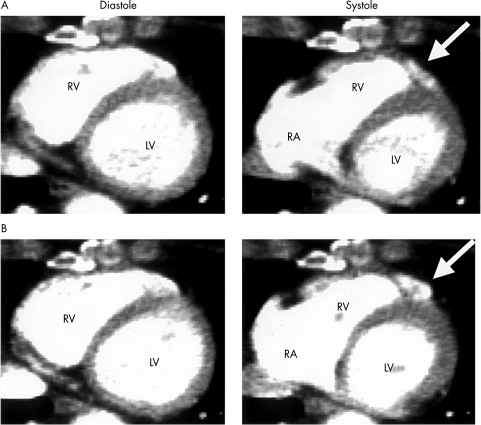
After the disopyramide infusion, there was deterioration in wall motion in eight patients (fig 1B). In two of them, the abnormalities were found only after the disopyramide infusion (fig 2). The sites of deterioration in the wall motion abnormalities were limited at the anterior base of the right ventricular outflow tract region. None of the patients in the unchanged group had diffuse deterioration of wall motion after the disopyramide infusion.
Figure 2.
Representative electron beam computed tomography findings in a patient in the “deteriorated” group (see text for definition of the groups) without wall motion abnormalities before the disopyramide infusion (A, arrowhead). The wall motion abnormalities were unmasked after the disopyramide infusion (B, arrowhead). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
EBT findings in the control subjects
None of the controls, with or without right bundle branch block, had abnormal findings in relation to wall thickness or the focal presence of fatty tissue on static images. In none of the controls were there wall motion abnormalities or deterioration of the wall motion after the disopyramide infusion.
Morphological assessment: assessment of the left ventricle
No abnormal left ventricular findings were observed in any of the patients with Brugada syndrome or in the control subjects, either before or after the disopyramide infusion. There were no significant differences in either interobserver or intraobserver variability between the two groups of patients and the controls.
Twelve lead ECG findings
QRS duration in patients with Brugada syndrome
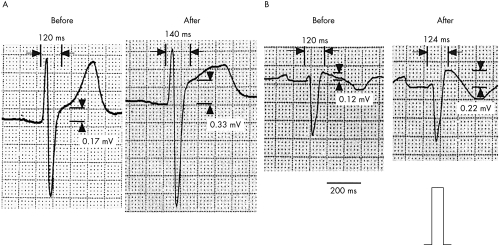
The mean QRS durations before and after the disopyramide infusion in the 13 patients with Brugada syndrome are presented in table 2. No significant difference between the two groups was present in any lead. After the disopyramide infusion, the mean QRS duration increased in both groups in all the leads (p < 0.05). In lead V2 only, the mean difference in the QRS duration compared with the pre-disopyramide value (mean ΔQRS duration) was larger in the deteriorated group than in the unchanged group (23 (5) v 10 (3) ms, respectively; p = 0.0001) (table 3). Representative ECG findings are shown in fig 3.
Table 2.
QRS duration before and after disopyramide infusion
| Before disopyramide | After disopyramide | Statistics (p value) | |||||
| Lead | Deteriorated (n=8) (ms) | Unchanged (n=5) (ms) | Deteriorated (n=8) (ms) | Unchanged (n=5) (ms) | Group factor | Drug factor | Interaction |
| I | 96 (25) | 86 (10) | 118 (31) | 96 (8) | 0.23 | <0.01* | 0.12 |
| II | 111 (25) | 105 (17) | 124 (29) | 112 (11) | 0.53 | 0.03* | 0.53 |
| III | 103 (16) | 110 (14) | 115 (18) | 121 (8) | 0.41 | <0.01* | 0.94 |
| aVR | 104 (27) | 101 (16) | 119 (26) | 113 (15) | 0.74 | <0.01* | 0.54 |
| aVL | 107 (27) | 94 (17) | 110 (27) | 105 (16) | 0.47 | <0.01* | 0.06 |
| aVF | 111 (27) | 111 (17) | 122 (27) | 116 (9) | 0.84 | <0.01* | 0.20 |
| V1 | 114 (16) | 104 (12) | 128 (14) | 114 (12) | 0.14 | <0.01* | 0.56 |
| V2 | 108 (13) | 100 (8) | 131 (15) | 110 (7) | 0.06 | <0.01* | <0.01* |
| V3 | 117 (16) | 106 (9) | 132 (13) | 114 (10) | 0.06 | <0.01* | 0.07 |
| V4 | 117 (22) | 102 (10) | 131 (20) | 113 (7) | 0.14 | <0.01* | 0.53 |
| V5 | 109 (21) | 103 (15) | 123 (18) | 111 (12) | 0.39 | <0.01* | 0.21 |
| V6 | 104 (21) | 102 (15) | 120 (25) | 112 (14) | 0.68 | <0.01* | 0.31 |
Values are mean (SD).
*Significant difference before and after disopyramide.
Table 3.
Difference in QRS duration before and after disopyramide infusion (ΔQRS duration)
| Lead | Deteriorated group (n=8) (ms) | Unchanged group (n=5) (ms) | p Value |
| I | 18 (11) | 12 (10) | 0.52 |
| II | 11 (10) | 9 (6) | 0.30 |
| III | 11 (6) | 12 (9) | 0.14 |
| aVR | 15 (9) | 13 (9) | 0.13 |
| aVL | 9 (4) | 8 (3) | 0.23 |
| aVF | 12 (9) | 9 (3) | 0.28 |
| V1 | 13 (8) | 12 (10) | 0.08 |
| V2 | 23 (5) | 10 (3) | <0.01 |
| V3 | 15 (7) | 8 (5) | 0.13 |
| V4 | 13 (10) | 12 (3) | 0.17 |
| V5 | 14 (8) | 9 (8) | 0.51 |
| V6 | 13 (6) | 12 (3) | 0.62 |
Values are mean (SD).
Figure 3.
Representative lead V2 ECGs from patients with Brugada syndrome before and after the disopyramide infusion. The difference in the QRS duration and magnitude of the ST segment elevation of that observed before the disopyramide infusion with that after was significantly larger in the patients in the “deteriorated” group (A) than in those in the “unchanged” group (B) (see text for definition of the groups).
ST segment elevation in lead V2 in patients with Brugada syndrome
Under control conditions, all patients showed ST segment elevation (coved or saddleback type in precordial leads V1–V3) during sinus rhythm. The maximum ST segment elevation occurred in lead V2. The mean ST segment elevation before disopyramide was 0.28 (0.24) mV in the deteriorated group and 0.27 (0.10) mV in the unchanged group. After disopyramide, the magnitude of the ST segment elevation increased in all patients (p = 0.001). Mean ST segment elevation after disopyramide was 0.51 (0.35) mV in the deteriorated group and 0.41 (0.08) mV in the unchanged group. There was no significant difference between the two groups in this variable.
The mean difference in the magnitude of the ST segment elevation before and after the disopyramide infusion (mean ΔST) was 0.24 (0.12) mV in the deteriorated group and 0.14 (0.07) mV in the unchanged group. The difference was greater in the patients in the deteriorated group than in those in the unchanged group, but this did not reach significance (p = 0.15). Representative ECG findings are shown in fig 3.
QRS duration and ST segment elevation in the control subjects without right bundle branch block
The mean QRS duration before disopyramide in the controls without right bundle branch block was less than in the patients with Brugada syndrome in all leads (p < 0.05). None of the control subjects had either pronounced ST segment elevation (more than 0.16 (0.02) mV in lead V2) or QTc prolongation (more than 377 (13) ms½ in lead V2) under control conditions.
After the disopyramide infusion, the patients in the unchanged group showed a larger mean ΔQRS duration (p = 0.17 in lead V2) and a larger mean ΔST than the control subjects. However, there were no significant difference in these variables between the patients in the unchanged group and the control subjects in any lead.
Electrophysiological study
No abnormal bipolar electrograms were recorded in the right ventricle in any patient. The mean HV intervals were 41 (7) ms and 38 (4) ms in the deteriorated group and unchanged group, respectively (NS). Ventricular fibrillation was induced in seven patients (88%) in the deteriorated group and in four patients (80%) in the unchanged group (NS) by double or triple extrastimuli from the right ventricular apex or outflow tract region (table 1).
DISCUSSION
Our findings show that wall motion abnormalities of the anterior base of the right ventricular outflow tract region can be detected in a substantial number (69%) of patients with Brugada syndrome using EBT under control conditions. Eight of our 13 patients with this syndrome had further deterioration of the wall motion in the anterior basal region of the right ventricular outflow tract after the disopyramide infusion. In two of these eight patients, the wall motion abnormalities were unmasked after the disopyramide infusion. We also found that patients with deterioration in wall motion after disopyramide had significant prolongation of QRS duration and a larger change in QRS duration in lead V2—which reflects the electrical activity of the right ventricular outflow tract region—than the patients with no impairment of wall motion after disopyramide. Thus we found that patients with the Brugada syndrome show heterogeneity of response to sodium channel blockers, and that the area with this heterogeneous response is in the right ventricular outflow tract region.
Although Brugada and colleagues found no clear underlying heart disease in their patients and proposed that the cause of the syndrome was an electrical disorder,1 some other studies7–10 have reported wall motion abnormalities of the right ventricle in patients with the Brugada syndrome. Martini and colleagues found morphological and wall motion abnormalities of the right ventricle in five of six patients, and observed right ventricular enlargement, significant fibrous adipose replacement in the free wall, and fibrosis of the bifurcating bundle and proximal bundle branches in one necropsy case.7 Corrado and associates reported that four of seven members of a family affected by the syndrome, and in whom characteristics including right bundle branch block, ST segment elevation, and sudden cardiac death were observed, had structural abnormalities of the right ventricle on echocardiography.9 A recent study carried out by us using EBT8 showed that patients with Brugada syndrome had a high incidence of wall motion abnormalities in the right ventricle, which suggest that this condition may be associated with underlying heart disease involving the right ventricle, especially the right ventricular outflow tract region. In some studies it has been found that class IA and IC sodium channel blockers may augment the ST segment elevation.2–5 Moreover, some mutations in the cardiac sodium channel gene SCN5A—a gene linked to Brugada syndrome—have recently been reported.6,13
In the present study, we found that some of our patients with Brugada syndrome showed further deterioration in wall motion in the right ventricular outflow tract region after disopyramide—that is, these patients may be more sensitive to sodium channel blockers in that area of the heart. As we found no wall motion abnormalities before or after disopyramide in the controls with right bundle branch block, we conclude that the observed abnormalities in wall motion are not caused by asynchrony of depolarisation but are specific to the Brugada syndrome. We suggest that in these patients the deterioration in the wall motion abnormalities detected by EBT may be functional and may be related to sodium channel abnormalities.
We believe this is the first study to demonstrate a relation between wall motion abnormalities and an abnormal response to sodium channel blockers, and that sodium channel abnormalities may be present in the right ventricular outflow tract region in patients with the Brugada syndrome. Other patients with this syndrome but without deterioration in the wall motion after disopyramide also showed a longer mean ΔQRS duration and a larger mean ΔST than the control subjects. We therefore suggest that patients with Brugada syndrome are likely to have an abnormal response to sodium channel blockers, but that EBT revealed deterioration in the wall motion after disopyramide only in those patients who had severe sodium channel abnormalities.
In the six patients with wall motion abnormalities at baseline and with deterioration in wall motion after disopyramide, we speculate that the baseline abnormalities may be a manifestation of a depolarisation abnormality based on a sodium channel defect, or they might indicate underlying structural heart disease. In the three patients with wall motion abnormalities but no deterioration after disopyramide, we assume there may be underlying structural heart disease, but the aetiology of this remains unclear. As the number of such patients was so small, we need more data on similar cases for a better understanding of the aetiology of wall motion abnormalities unresponsive to disopyramide.
In our study, the mean difference in the magnitude of the ST segment before and after disopyramide was greater in the deteriorated group than in the unchanged group, but this difference was not significant. Some basic studies of experimental models of the Brugada syndrome have suggested that ST segment elevation is related to an increased transient outward current (Ito) in the right ventricular epicardium but not in the right ventricular endocardium.14,15 Disopyramide has modest Ito blocking properties16 and these could mask any differences in ST segment elevation.
Study limitations
Flecainide, a class Ic sodium channel blocker, is widely used in provocative testing to unmask the ECG phenotype of Brugada syndrome because of its strong use dependent blockade of the fast sodium current.17,18 We initially used flecainide in a few of our patients; however, on EBT all these patients showed diffuse deterioration in wall motion after the infusion, probably because of the strong negative inotropic effect of this agent. Because of this, we were unable to assess the specific changes in wall motion abnormality. In the present study, therefore, we used disopyramide as a sodium channel blocker, because of its weaker negative inotropic effects.
We undertook genetic analyses on 10 patients but did not identify any genetic disorders. As the number of patients was so small, further data are needed for a better understanding of relation between gene mutation and focal wall motion abnormalities in patients with the Brugada syndrome.
In order to evaluate whether the presence or absence of the wall motion abnormalities observed has prognostic relevance, we need to collect prospective data on spontaneously occurring ventricular tachyarrhythmias.
Conclusions
Deterioration in wall motion abnormalities in the right ventricular outflow tract region after a disopyramide infusion suggests the presence of functional abnormalities of the sodium channel. This means that some patients with Brugada syndrome may have arrhythmogenic substrates with heterogeneous responses to sodium channel blockers in the right ventricular outflow tract region.
REFERENCES
- 1.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation 1998;97:457–60. [DOI] [PubMed] [Google Scholar]
- 2.Brugada J, Brugada P. Further characterization of the syndrome of right bundle branch block, ST segment elevation, and sudden cardiac death. J Cardiovasc Electrophysiol 1997;8:325–31. [DOI] [PubMed] [Google Scholar]
- 3.Miyazaki T, Mitamura H, Miyoshi S, et al. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol 1996;27:1061–70. [DOI] [PubMed] [Google Scholar]
- 4.Krishman SC, Josephson ME. ST segment elevation induced by class IC antiarrhythmic agents: Underlying electrophysiological mechanisms and insights into drug-induced proarrhythmia. J Cardiovasc Electrophysiol 1998;9:1167–72. [DOI] [PubMed] [Google Scholar]
- 5.Fujiki A, Usui M, Nagasawa H, et al. ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insights into the mechanism of Brugada syndrome. J Cardiovasc Electrophysiol 1999;10:214–18. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Kirsch GE, Zhang D, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998;392:293–6. [DOI] [PubMed] [Google Scholar]
- 7.Martini B, Nava A, Thiene G, et al. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J 1989;118:1203–9. [DOI] [PubMed] [Google Scholar]
- 8.Takagi M, Aihara N, Kuribayashi S, et al. Localized right ventricular morphological abnormalities detected by electron-beam computed tomography represent arrhythmogenic substrates in patients with Brugada syndrome. Eur Heart J 2001;22:1032–41. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D, Nava A, Buja G, et al. Familial cardiomyopathy underlies syndrome of right bundle branch block, ST segment elevation and sudden death. J Am Coll Cardiol 1996;27:443–8. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Kato K, Hashimoto M, et al. Localized right ventricular structural abnormalities in patients with idiopathic ventricular fibrillation: magnetic resonance imaging study. Heart Vessels 1996;11:100–3. [DOI] [PubMed] [Google Scholar]
- 11.Tada H, Shimizu W, Ohe T, et al. Usefulness of electron–beam computed tomography in arrhythmogenic right ventricular dysplasia: relationship to electrophysiological abnormalities and left ventricular involvement. Circulation 1996;94:437–44. [DOI] [PubMed] [Google Scholar]
- 12.Hamada S, Takamiya M, Ohe T, et al. Arrhythmogenic right ventricular dysplasia: evaluation with electron-beam CT. Radiology 1993;187:723–7. [DOI] [PubMed] [Google Scholar]
- 13.Makita N, Shirai N, Wang DW, et al. Cardiac Na+ channel dysfunction in Brugada syndrome is aggravated by β-subunit. Circulation 2000;101:54–60. [DOI] [PubMed] [Google Scholar]
- 14.Antzelevitch C, Yan GX, Shimizu W. Electrical heterogeneity, the ECG, and cardiac arrhythmias. In: Zipes DP, Jalife J, eds. Cardiac electrophysiology: from cell to bedside. Philadelphia: WB Saunders Co, 2000:222–38.
- 15.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation 1996;93:372–9. [DOI] [PubMed] [Google Scholar]
- 16.Virag L, Varro A, Papp C. Effect of disopyramide on potassium currents in rabbit ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 1998;357:268–75. [DOI] [PubMed] [Google Scholar]
- 17.Brugada R, Brugada J, Anzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation 2000;101:510–15. [DOI] [PubMed] [Google Scholar]
- 18.Priori SG, Napolitano C, Schwartz PJ, et al. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation 2000;102:945–7. [DOI] [PubMed] [Google Scholar]