Abstract
Objective: To determine whether the process of reverse left ventricular remodelling in response to carvedilol is dependent on baseline heart rate (BHR), heart rhythm, or heart rate reduction (HRR) in response to carvedilol.
Design: Retrospective analysis of serial echocardiograms in 257 patients with chronic systolic heart failure at baseline and at 12–18 months after starting carvedilol. Reverse left ventricular remodelling was determined by changes in left ventricular end diastolic dimension (LVEDD), end systolic dimension (LVESD), and fractional shortening (LVFS).
Setting: Heart failure clinic within a university teaching hospital.
Main outcome measures: Changes in LVEDD, LVESD, and LVFS.
Results: LVEDD and LVESD decreased by 2.6 (0.4) mm and 4.9 (0.5) mm, respectively (mean (SEM)), and LVFS increased by 4.3 (0.5)% (all p < 0.0001 v baseline). Simple regression revealed no significant relation between BHR or HRR and the changes in LVEDD, LVESD, or LVFS. Stratification of patients into high and low BHR groups (above and below the mean) or according to the baseline heart rhythm (sinus rhythm v atrial fibrillation) showed no differences between groups in the extent of reverse left ventricular remodelling. Improvements in left ventricular function and dimensions were associated with significant improvements in New York Heart Association functional class.
Conclusions: The benefits of carvedilol in terms of reverse left ventricular remodelling and symptomatic improvement in patients with chronic heart failure are independent of BHR, heart rhythm, and the HRR that occurs in response to carvedilol.
Keywords: β blocker, carvedilol, heart failure, remodelling
The importance of baseline heart rate (BHR) and treatment induced heart rate reduction (HRR) as predictors of the response to β blockers in chronic heart failure is controversial. Some investigators have reported that the BHR is predictive of symptomatic improvement1 and improvement in left ventricular function in response to β blockade,2 while others have found no such relations.3–5 Post hoc stratification of patients in the US carvedilol heart failure programme6 into groups above and below the mean BHR showed that the mortality benefit of carvedilol compared with placebo was only significant in the group with a high BHR. Recently, the CIBIS II (cardiac insufficiency bisoprolol study) investigators7 confirmed that BHR was an independent predictor of mortality in patients with chronic heart failure. The benefit of bisoprolol in terms of survival and the need for hospital admission was, however, independent of the BHR and the HRR that occurred during the first two months of bisoprolol treatment.7 In addition, these investigator reported that the beneficial response to bisoprolol was observed only in patients in sinus rhythm and not in those with atrial fibrillation. They suggested that baseline heart rhythm rather than heart rate may be an important determinant of the response to β blocker treatment in heart failure.
In order to investigate further the importance of BHR, cardiac rhythm, and HRR in determining the response to β blocker treatment in chronic heart failure, we analysed the relation between these variables and echocardiographic left ventricular remodelling in response to carvedilol in a large consecutive cohort of patients attending a heart failure clinic.
METHODS
Study population
The study population was derived from 429 consecutive patients who were started on carvedilol for chronic systolic heart failure at our institution and who had been followed for at least 12 months thereafter. All patients had been receiving treatment for heart failure for at least three months before starting carvedilol. One hundred and six patients (25%) were withdrawn from carvedilol treatment within the first 12 months because of death (32), heart transplantation (28), or other serious adverse events (46). Another 66 patients were excluded from the echocardiographic analysis: 52 (12%) had technically unsatisfactory echocardiographic studies, and 14 (3%) did not undergo a second scan. Reverse left ventricular remodelling was assessed in the remaining 257 patients (60%) who underwent serial echocardiograms at baseline and between 12–18 months after starting carvedilol.
Carvedilol administration
Carvedilol was begun at a dose of 3.125 mg twice daily and was force titrated, at two weekly intervals, to a target dose of 25 mg twice daily for body weight ≤ 85 kg, or 50 mg twice daily for body weight > 85 kg, as tolerated.
Assessment and follow up
All patients were assessed clinically at baseline and at three, six, and 12 months, and then at six monthly intervals. Clinical status was assessed at each visit, including evaluation of New York Heart Association (NYHA) functional class, resting heart rate and rhythm, blood pressure, and cardiac examination. Echocardiograms were done at baseline and at the 12 or 18 month visits. Reverse left ventricular remodelling was determined by measuring the changes in left ventricular end diastolic dimension (LVEDD), left ventricular end systolic dimension (LVESD), and left ventricular fractional shortening (LVFS) between the baseline and follow up echocardiograms. Measurements of left ventricular dimensions and calculation of fractional shortening were made using cross sectionally guided M mode echocardiography according to the American Society of Echocardiography standards,8 as described previously.9,10 Measurements were made from the average of three cardiac cycles for patients in sinus rhythm and from an average of 10 cycles for patients in atrial fibrillation. All measurements were made by one of two technicians who were blinded to the treatment status of the patient.
Statistical analysis
Data are presented as mean (SEM). Baseline demographic data were compared using either factorial analysis of variance or χ2 analyses for multiple groups (table 1), and unpaired t tests or χ2 analyses for high and low BHR groups (table 2). The Bonferroni correction was used to adjust for multiple comparisons. The relation between reverse left ventricular remodelling and BHR was examined by treating BHR as both a continuous variable and a categorical variable, in the latter case by dividing patients into two groups above and below the mean BHR. Changes in left ventricular size and function in relation to BHR were compared using simple regression for continuous variables and by unpaired t tests for comparisons between categorical variables. The heart rate reduction in response to carvedilol (HRR) was determined by subtracting the resting heart rate measured at the time of follow up echocardiography from the baseline heart rate. The relation between changes in left ventricular size and function and HRR was examined by simple regression. A probability value of p < 0.05 with Bonferroni correction was considered significant.
Table 1.
Baseline characteristics of all patients started on carvedilol
| Withdrawn patients (n=106) | Excluded patients (n=66) | Included patients (n=257) | |
| Mean age (years) | 60 | 61 | 57 |
| Sex (male:female) (%) | 85:15 | 93:7 | 86:14 |
| Diagnosis: IHD/CM/other (%) | 37/56/7 | 39/53/8 | 30/65/5**** |
| Duration of heart failure (months) | 40 | 37 | 45 |
| Atrial fibrillation (%) | 16 | 24 | 18 |
| Heart rate (beats/min) | 80 | 81 | 82 |
| Systolic blood pressure (mm Hg) | 110 | 119 | 114 |
| Serum sodium (mmol/l) | 138 | 138 | 138 |
| Serum creatinine (mmol/l) | 0.13 | 0.12 | 0.12 |
| 6 min walk distance (m) | 395† | 472 | 447 |
| NYHA class: I/II/III/IV (%) | 1/11/53/37*** | 2/21/61/16 | 6/26/48/20 |
| Drugs (%) | |||
| ACE inhibitors | 94 | 98 | 97 |
| Diuretics | 96 | 92 | 90 |
| Digoxin | 79 | 77 | 76 |
| Amiodarone | 41 | 52 | 32 |
| Echocardiographic variables | |||
| LVEDD (mm) | 72 | 74 | 73 |
| LVESD (mm) | 61 | 62 | 63 |
| LVFS (%) | 15 | 17 | 15 |
***p < 0.001 v other groups; ****p < 0.0001 v other groups.
ACE, angiotensin converting enzyme; CM, cardiomyopathy; IHD, ischaemic heart disease; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVFS, left ventricular fractional shortening; NYHA, New York Heart Association.
Table 2.
Baseline characteristics of patients included in echocardiographic analysis stratified into groups above and below the mean baseline heart rate of 82 beats/min
| Variable | High BHR (n=123) | Low BHR (n=134) | p Value |
| Age (years) | 56 (1) | 58 (1) | 0.18 |
| Sex (male:female) (%) | 83:17 | 88:12 | 0.22 |
| Diagnosis: IHD/CM/other (%) | 29/67/4 | 31/62/7 | 0.63 |
| Duration of heart failure (months) | 43 (4) | 47 (4) | 0.45 |
| Atrial fibrillation (%) | 13 | 23 | 0.17 |
| Heart rate (beats/min) | 93 (1) | 72 (1) | <0.0001 |
| Systolic blood pressure (mm Hg) | 113 (3) | 114 (2) | 0.78 |
| Serum sodium (mmol/l) | 138 (1) | 138 (1) | 0.56 |
| Serum creatinine (mmol/l) | 0.125 (0.010) | 0.114 (0.003) | 0.29 |
| 6 min walk distance (m) | 433 (12) | 460 (11) | 0.11 |
| NYHA class: I/II/III/IV (%) | 7/23/46/24 | 4/29/51/16 | 0.23 |
| Drug treatment (%) | |||
| ACE inhibitors | 96 | 98 | 0.41 |
| Diuretics | 89 | 90 | 0.86 |
| Digoxin | 72 | 79 | 0.19 |
| Amiodarone | 26 | 37 | 0.07 |
| Echocardiographic variables | |||
| LVEDD (mm) | 73 (1) | 74 (1) | 0.56 |
| LVESD (mm) | 63 (1) | 63 (1) | 0.94 |
| LVFS (%) | 14 (1) | 15 (1) | 0.10 |
Values are % or mean (SEM).
ACE, angiotensin converting enzyme; BHR, baseline heart rate; CM, cardiomyopathy; IHD, ischaemic heart disease; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVFS, left ventricular fractional shortening; NYHA, New York Heart Association.
RESULTS
Patient population
The baseline characteristics of the 429 patients who were started on carvedilol are summarised in table 1. Patients were divided into three groups: those withdrawn from carvedilol within the first 12 months (106), those excluded from the echocardiographic analysis owing to inadequate or missing scans (66), and those included in the echocardiographic analysis (257). Patients included in the echocardiographic analysis were more likely to have non-ischaemic dilated cardiomyopathy as the cause of their heart failure than those in the other two groups. Patients withdrawn from carvedilol within the first 12 months were more likely to be in NYHA functional class IV at baseline. In addition, their mean six minute walk distance was significantly lower than in the other groups. Overall, 19% of patients were in atrial fibrillation at baseline, with no significant difference across the three groups.
The 257 patients included in the echocardiographic analysis were divided into two groups above and below the mean BHR of 82 beats/min. There were no significant baseline differences between these two groups in terms of diagnosis, functional status, concomitant drug treatment, or echocardiographic indices (table 2). There were trends towards a higher prevalence of atrial fibrillation and greater use of amiodarone in the low BHR group, but these did not reach significance.
Forty five of the 257 patients (18%) were in atrial fibrillation at the start of carvedilol treatment. They were significantly older than patients in sinus rhythm (63 (1) v 56 (1) years, p < 0.001) and were more likely to have valvar or ischaemic heart disease as the antecedent cardiac diagnosis. Patients in atrial fibrillation had a lower baseline heart rate than those in sinus rhythm (79 (2) v 83 (1) beats/min, p < 0.05). The proportions of patients in atrial fibrillation who were receiving digoxin and amiodarone at the start of carvedilol treatment were higher than the corresponding proportions of patients in sinus rhythm (89% v 74% receiving digoxin, p < 0.05; 51% v 28% receiving amiodarone, p < 0.001).
Maintenance carvedilol dose and changes in vital signs
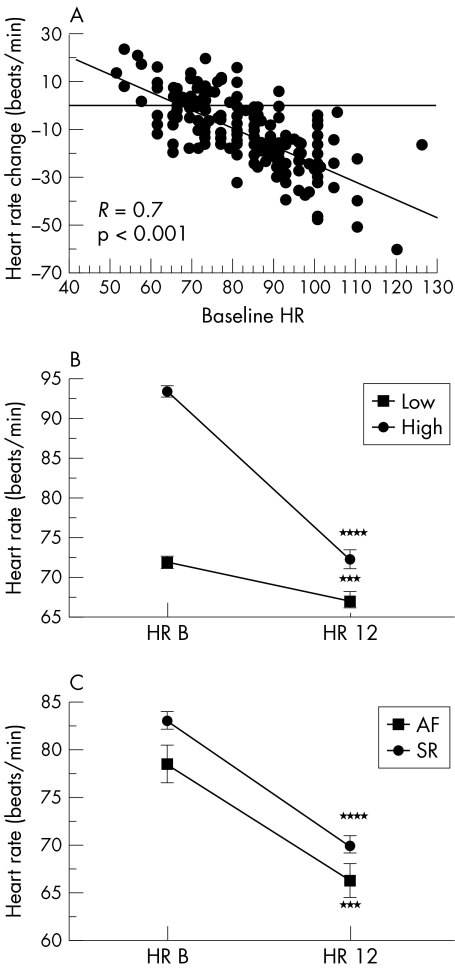
The mean maintenance dose of carvedilol at the time of follow up echocardiography was 39 (1) mg/day. The maintenance dose was similar in the high and low BHR groups, at 40 (2) v 38 (2) mg/day (not significant (NS)). HRR during carvedilol treatment is shown in fig 1A. Heart rate fell by a mean of 12 (1) beats/min (p < 0.0001 v baseline). There was a highly significant correlation between BHR and HRR (fig 1A), such that the higher the BHR the greater the HRR at 12 months. The HRR during carvedilol treatment was significant in both high and low BHR groups, but was much greater in the high BHR group: 20 (1) v 5 (1) beats/min (p < 0.0001, high BHR v low BHR) (fig 1B). In spite of this, the mean heart rate at follow up remained significantly lower in the low BHR group, at 67 (1) v 72 (1) beats/min (p = 0.001). There was a small, non-significant change in resting recumbent systolic blood pressure during carvedilol treatment (data not shown).
Figure 1.
Relation between heart rate reduction, baseline heart rate, and heart rhythm. (A) Linear regression plot of heart rate change after 12 months of carvedilol v baseline heart rate (BHR). (B) and (C) Mean heart rate (with standard error bars) measured at baseline (HR B) and after 12 months of carvedilol (HR 12), stratified according to high and low BHR groups (B) and according to baseline rhythm (C). AF, atrial fibrillation; SR, sinus rhythm. ***p < 0.001 v baseline; ****p < 0.0001 v baseline.
The mean maintenance dose of carvedilol at the time of follow up echocardiography was similar in patients in atrial fibrillation (38 (4) mg/day) and in sinus rhythm (39 (1) mg/day). Mean heart rate fell by 10 (2) beats/min in response to carvedilol in patients with atrial fibrillation (p < 0.001 v baseline) and by 13 (1) beats/min in patients in sinus rhythm (p < 0.0001 v baseline; NS compared with patients in atrial fibrillation) (fig 1C).
Reverse left ventricular remodelling: echocardiographic changes in relation to BHR, HRR, and heart rhythm
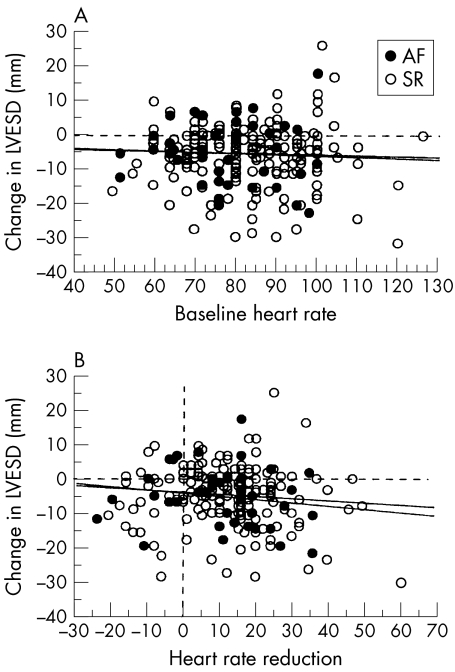
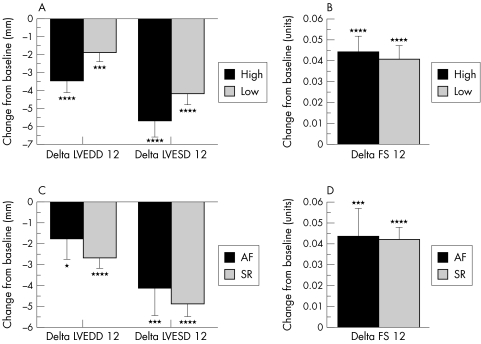
There were highly significant falls in LVEDD and LVESD over 12–18 months in response to carvedilol. LVEDD fell by a mean of 2.6 (0.4) mm (p < 0.0001 v baseline) and LVESD fell by 4.9 (0.5) mm (p < 0.0001 v baseline). LVFS rose by 4.3 (0.5)% (p < 0.0001 v baseline). These changes in echocardiographic variables occurred independently of BHR and HRR. Simple regression analyses showed no significant association between changes in echocardiographic variables (dependent variables) and baseline heart rate or heart rate reduction (independent variables), irrespective of whether the patient was in sinus rhythm (n = 212) or atrial fibrillation (n = 45). The regression coefficients with corresponding p values for these analyses are given in table 3. Figure 2 shows illustrative scatter plots for the change in LVESD plotted against BHR (fig 2A) and HRR (fig 2B). Figure 3 illustrates the mean changes in these same echocardiographic indices expressed as a categorical variable. Left ventricular dimensions (fig 3A) and fractional shortening (fig 3B) improved significantly over time in both high and low BHR groups, but there were no differences between the two groups. Separate stratification of patients into two groups with a BHR ≤ 70 beats/min (n = 55) or > 70 beats/min (n = 202) also failed to reveal any differences in the degree of reverse left ventricular remodelling between the groups (data not shown). Similarly, stratification of patients into two groups according to the baseline rhythm (sinus rhythm (n = 212) v atrial fibrillation (n = 45)) showed no significant differences between the groups with regard to changes in LVEDD, LVESD (fig 3C), or LVFS (fig 3D).
Table 3.
Regression coefficients with corresponding probability values for regression analyses between changes in echocardiographic variables plotted against baseline heart rate or heart rate reduction
| Sinus rhythm (n=212) | Atrial fibrillation (n=45) | |||
| Echocardiographic variable | IRI | p Value | IRI | p Value |
| Baseline heart rate | ||||
| Change in LVEDD | 0.156 | 0.03 | 0.198 | 0.22 |
| Change in LVESD | 0.051 | 0.48 | 0.032 | 0.84 |
| Change in LVFS | 0.096 | 0.19 | 0.254 | 0.12 |
| Heart rate reduction | ||||
| Change in LVEDD | 0.062 | 0.42 | 0.041 | 0.81 |
| Change in LVESD | 0.093 | 0.23 | 0.152 | 0.37 |
| Change in LVFS | 0.056 | 0.46 | 0.258 | 0.12 |
Applying a Bonferroni correction, a p value of < 0.004 was considered significant.
IRI, regression coefficient; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVFS, left ventricular fractional shortening.
Figure 2.
Scatterplots showing the relations between changes in LVESD and baseline heart rate (A) and heart rate reduction (B). No significant associations were identified for any of the plotted variables. AF, atrial fibrillation; LVESD, left ventricular end systolic dimension; SR, sinus rhythm.
Figure 3.
Histograms showing changes in values of echocardiographic variables between baseline and follow up echocardiograms, stratified into high and low baseline heart rate (BHR) groups (panels A and B) and into atrial fibrillation (AF) and sinus rhythm (SR) groups (panels C and D). Data are plotted as means with standard errors. AF, atrial fibrillation; LVESD, left ventricular end systolic dimension; SR, sinus rhythm. *p < 0.1 v baseline; ***p < 0.005 v baseline; ****p < 0.0001 v baseline. There were no significant differences between groups for any of the measured variables.
Clinical outcome
One hundred and six of the 429 patients (25%) were withdrawn from carvedilol within the first 12 months. The reasons for withdrawal were death in 32 patients (7%), heart transplantation in 28 (7%), and serious adverse events in 46 (11%). Symptomatic bradycardia was reported by 13 patients (3%) and led to withdrawal of carvedilol in seven (2%) Two of these patients had a BHR above the group mean and five had a BHR below the mean (NS). The BHR of patients withdrawn because of bradycardia was 73 (4) beats/min, compared with 82 (1) beats/min for the remainder of the study population (p = 0.10).
Of the 257 patients included in the echocardiographic analysis, 52% were assessed as being in a better NYHA class at the time of follow up echocardiography, 45% were unchanged, and only 3% were assessed as being in a worse class (p < 0.0001 v baseline). Patients whose NYHA class improved had significantly greater improvements in LVESD and LVFS than those whose NYHA class was unchanged or worse (table 4). Stratification of patients into low and high BHR groups revealed no significant differences in clinical outcome (table 5), nor did stratification according to baseline cardiac rhythm (table 5).
Table 4.
Changes in NYHA functional class in relation to changes in echocardiographic variables
| Change in NYHA class | Change in LVEDD (mm) | Change in LVESD (mm) | Change in LVFS (%) |
| Improved | −3.1 (0.7) | −6.6 (0.8)** | 6.2 (0.7)*** |
| Unchanged | −2.0 (0.6) | −3.1 (0.7) | 2.4 (0.7) |
| Worse | −2.1 (2.2) | −1.8 (2.8) | 0.5 (3.3) |
**p < 0.005 v other groups; ***p < 0.001 v other groups.
LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LVFS, left ventricular fractional shortening; NYHA, New York Heart Association.
Table 5.
Changes in NYHA functional class in relation to basal heart rate and rhythm
| Change in NYHA class | Low BHR (n=123) | High BHR (n=134) | AF (n=45) | SR (n=212) |
| Improved | 62 | 70 | 28 | 104 |
| Unchanged | 69 | 48 | 17 | 100 |
| Worse | 4 | 4 | 0 | 8 |
Values are numbers of patients.
AF, atrial fibrillation; BHR, basal heart rate; NYHA, New York Heart Association; SR, sinus rhythm.
DISCUSSION
Relation between BHR, HRR, and response to drug treatment in chronic heart failure
BHR has been reported to be an important determinant of the response to enalapril11 and amiodarone12 in patients with severe heart failure. In both studies, the benefit of these drugs in terms of survival was confined to patients with a raised BHR11,12 and was associated with a significant HRR in these patients. The importance of BHR and HRR as predictors of the response to β blockers in patients with heart failure is controversial. There is evidence that HRR mediates the benefit of β blockade on left ventricular contractility in experimental heart failure associated with a pronounced elevation in the BHR.13 In that study, chronic administration of atenolol to dogs with heart failure resulted in a significant improvement in left ventricular contractility. This improvement could be prevented completely by chronic pacing at a rate equal to the pretreatment heart rate. The relevance of this model to the majority of patients with heart failure can be questioned, however, as all animals had a pronounced increase in BHR before treatment with atenolol. Furthermore, the heart failure was induced by creating severe mitral regurgitation, which is an uncommon cause of heart failure in humans.
Clinical studies have produced conflicting results. Some clinical investigators have reported that the BHR is predictive of symptomatic improvement1 and improvement in left ventricular function in response to β blockade,2 while others have failed to find such a relation.3–5 Lechat and colleagues reported that HRR measured two months after starting bisoprolol was the single most powerful predictor of survival in the CIBIS I study.5 In contrast, these investigators found that the benefit of bisoprolol in terms of survival and the need for hospital admission was completely independent of HRR in the much larger CIBIS II study.7 Retrospective analysis of the CIBIS II study did confirm that BHR was an independent predictor of mortality in patients with chronic heart failure; however, the mortality benefit of bisoprolol was independent of the BHR. A similar finding was reported in the MERIT (metoprolol CR/XL randomised intervention trial) study.14 In that study, stratification of patients into lower versus middle and upper tertiles for BHR revealed no differences between groups in the mortality benefit obtained in response to metoprolol. On the other hand, stratification of patients in the US carvedilol study6 into groups above and below the mean BHR suggested a greater survival benefit from carvedilol in the high BHR group (although the difference between groups was not significant).
Relation between BHR, HRR, and reverse left ventricular remodelling in response to carvedilol
The primary findings of our study were that the improvements in left ventricular size and function that occurred in response to carvedilol were independent of the BHR (regardless of whether BHR was treated as a continuous or a categorical variable), or of the HRR or heart rhythm. Our findings regarding the lack of an association between reverse left ventricular remodelling, BHR, and HRR are consistent with and complement the findings of the CIBIS II study.7 Taken together, these findings indicate that HRR by itself cannot explain the reverse left ventricular remodelling and survival benefit produced by β blockers in patients with chronic heart failure.
Several mechanisms other than HRR have been proposed to explain the clinical benefits of β blockers in heart failure. These include improved diastolic function15 and inhibition of catecholamine induced myocyte necrosis16 and apoptosis.17,18 Which if any of these mechanisms is clinically important cannot be determined from our study. However, our findings and those of other investigators strongly suggest that reverse left ventricular remodelling is an important mediator of the clinical benefit of β blockers.5,19,20 This conclusion is further supported by our observation and that of other investigators that improvements in symptomatic status and survival are correlated closely with reverse left ventricular remodelling.5,19,20
Relation between heart rhythm and reverse left ventricular remodelling
Atrial fibrillation is a common arrhythmia in patients with heart failure. Eighteen per cent of patients in our study were in atrial fibrillation at the start of carvedilol treatment. This proportion is similar to the proportions reported in the MERIT and CIBIS II studies.7,14 In our study, we did not observe any significant difference in the extent of reverse left ventricular remodelling or symptomatic improvement between patients in sinus rhythm and those with atrial fibrillation. An unexpected finding of the CIBIS II study was the apparent lack of mortality benefit from bisoprolol in patients with atrial fibrillation.7 This may simply reflect a type II error owing to the relatively small numbers of patients with atrial fibrillation. Pooling of data from other β blocker trials in heart failure—as suggested by the CIBIS II investigators—would be very useful in confirming or refuting the hypothesis that the presence of atrial fibrillation abrogates the survival benefit of β blockers in heart failure. Our own findings suggest that patients with atrial fibrillation derive as much benefit from carvedilol as those in sinus rhythm.
Relation between BHR and tolerance to carvedilol
Krum and colleagues recently reported that patients with a BHR less than 70 beats/min showed similar tolerance to carvedilol as patients with a higher BHR.21 The incidence of symptomatic bradycardia was very low in our series and led to withdrawal of carvedilol in only 2% of patients. Furthermore, the mean BHR of patients withdrawn from carvedilol because of bradycardia was not statistically lower than the BHR for the group as a whole. One possible explanation for the excellent tolerance to carvedilol among patients with a low BHR is the relation between BHR and HRR in response to carvedilol (fig 1A). It is apparent from this relation that the lower the BHR, the smaller the HRR in response to carvedilol. While this relation could be explained by regression to the mean, it is noteworthy that a similar relation between BHR and HRR was reported in the CIBIS I study in response to bisoprolol.5
Conclusions
The benefits of carvedilol in terms of reverse left ventricular remodelling and symptomatic improvement in patients with chronic heart failure are independent of BHR, heart rhythm, and the heart rate reduction that occurs in response to carvedilol. The magnitude of the latter is directly related to the resting heart rate.
Acknowledgments
We wish to thank Associate Professor Henry Krum for reviewing the manuscript and for his suggestions regarding its final format.
Abbreviations
BHR, baseline heart rate
CIBIS, cardiac insufficiency bisoprolol study
HRR, heart rate reduction
LVEDD, left ventricular end diastolic dimension
LVESD, left ventricular end systolic dimension
LVFS, left ventricular fractional shortening
MERIT, metoprolol CR/XL randomised intervention trial
NYHA, New York Heart Association
REFERENCES
- 1.DiLenarda A, Gregori D, Sinagra G, et al. Metoprolol in dilated cardiomyopathy: is it possible to identify factors predictive of improvement? The heart muscle disease study group. J Card Fail 1996;2:87–102. [DOI] [PubMed] [Google Scholar]
- 2.Schleman KA, Lindenfeld JA, Lowes BD, et al. Predicting response to carvedilol for the treatment of heart failure: a multivariate retrospective analysis. J Card Fail 2001;7:4–12. [DOI] [PubMed] [Google Scholar]
- 3.Metra M, Nardi M, Giubbini R, et al. Effects of short- and long-term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1994;24:1678–87. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorn EJ, Heesch CM, Risser RC, et al. Predictors of systolic and diastolic improvement in patients with dilated cardiomyopathy treated with metoprolol. J Am Coll Cardiol 1995;25:154–62. [DOI] [PubMed] [Google Scholar]
- 5.Lechat P, Escolano S, Golmard JL, et al. Prognostic value of bisoprolol-induced hemodynamic effects in heart failure during the cardiac insufficiency bisoprolol study (CIBIS). Circulation 1997;96:2197–205. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349–55. [DOI] [PubMed] [Google Scholar]
- 7.Lechat P, Hulot JS, Escolano S, et al. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 2001;103:1428–33. [DOI] [PubMed] [Google Scholar]
- 8.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald PS, Keogh AM, Aboyoun CL, et al. Tolerability and efficacy of carvedilol in patients with New York Heart Association class IV heart failure. J Am Coll Cardiol 1999;33:924–31. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald PS, Keogh AM, Aboyoun C, et al. Impact of concurrent amiodarone treatment on the tolerability and efficacy of carvedilol in patients with chronic heart failure. Heart 1999;82:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swedberg K, Eneroth P, Kjekshus J, et al. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation 1990;82:1730–6. [DOI] [PubMed] [Google Scholar]
- 12.Nul DR, Doval HC, Grancelli HO, et al.. Heart rate is a marker of amiodarone mortality reduction in severe heart failure. The GESICA-GEMA Investigators. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina-Grupo de Estudios Multicentricos en Argentina. J Am Coll Cardiol 1997;29:1199–205. [DOI] [PubMed] [Google Scholar]
- 13.Nagatsu M, Spinale FG, Koide M, et al. Bradycardia and the role of beta-blockade in the amelioration of left ventricular dysfunction. Circulation 2000;101:653–9. [DOI] [PubMed] [Google Scholar]
- 14.Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 15.Eichhorn EJ, Grayburn PA. Beta-blocker improvement in diastolic performance: the yin and yang of ventricular function changes. Am Heart J 2000;139:584–6. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshank JM, Neil-Dwyer G, Degaute JP, et al. Reduction of stress/catecholamine-induced cardiac necrosis by beta 1-selective blockade. Lancet 1987;ii:585–9. [DOI] [PubMed] [Google Scholar]
- 17.Singh K, Communal C, Sawyer DB, et al. Adrenergic regulation of myocardial apoptosis. Cardiovasc Res 2000;45:713–19. [DOI] [PubMed] [Google Scholar]
- 18.Romeo F, Li D, Shi M, et al. Carvedilol prevents epinephrine-induced apoptosis in human coronary artery endothelial cells: modulation of Fas/Fas ligand and caspase-3 pathway. Cardiovasc Res 2000;45:788–94. [DOI] [PubMed] [Google Scholar]
- 19.Doughty RN, Whalley GA, Gamble G, et al. Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand heart failure research collaborative group. J Am Coll Cardiol 1997;29:1060–6. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe N, Doughty RN. Left ventricular remodelling and improved long-term outcomes in chronic heart failure. Eur Heart J 1998;19(suppl B):B36–9. [PubMed] [Google Scholar]
- 21.Krum H, Ninio D, MacDonald P. Baseline predictors of tolerability to carvedilol in patients with chronic heart failure. Heart 2000;84:615–19. [DOI] [PMC free article] [PubMed] [Google Scholar]