In vivo hearts show fine beat to beat temporal variation during normal sinus rhythm (NSR). These changes have been termed “heart rate variability” (HRV). Accurate measurements of the beat to beat variation have enable investigators to determine the parameters of HRV.1 Routine assessment of HRV is derived from measurements of QRS-to-QRS (RR) intervals in the surface ECG. Activation of the heart starts with impulse formation in the sinus node and ends with excitation of the ventricular myocardium resulting in a QRS complex. Therefore, RR interval variation is likely to be the result of both sinus and atrioventricular (AV) node action. However, the relative contribution of each structure to HRV is not known.
We hypothesised that, by calculating and comparing HRV during NSR and different pacing manoeuvres, we would be able to elucidate the relative contribution of both nodes to HRV. Therefore, we measured and compared HRV during AAI pacing (elimination of impulse formation in the sinus node) and VAT pacing (elimination of contribution of the AV node).
METHODS
Twenty consecutive patients were studied during diagnostic electrophysiological testing. Informed written consent was obtained from all subjects. A time period of 600 seconds was used to calculate HRV during each pacing manoeuvre and NSR. HRV of NSR was calculated twice before and after the pacing manoeuvres. AAI pacing was done at a pacing rate 15% faster than the sinus rate, and VAT pacing with an AV delay of 80 ms. For pacing manoeuvres, an external AV sequential pacemaker was used. In addition DDD pacing 15% faster than the sinus rate with an AV delay of 80 ms was performed in all patients to assess measurement errors and noise ratio. Theoretically, calculations of HRV in the latter setting should result in zero. The Corazonix Predictor system was used for measurement of RR intervals. Extrasystoles and all non-captured beats during pacing were eliminated from further analysis including the preceding and succeeding cycles. Thereafter, mean heart rate (HR), standard deviation of all RR intervals (SDNN), and the square root of the mean of the squared successive differences in RR intervals (RMSSD) were calculated. Moreover, spectral parameters describing HRV were calculated for each of the four periods. Using a fast Fourier transformation (Hanning window) we evaluated the total (0–0.5 Hz), high frequency (0.15–0.5 Hz), low frequency (0.04–0.15 Hz), and very low frequency (0–0.04 Hz) power spectra.2
For calculations of the time domain parameters we used a commercially available software package (PCS V2, TopSoft, Hannover, Germany) and for statistical evaluation we used S-PLUS V3.4 (MathSoft Incorp, now Insightful Corp, Seattle, Washington, USA). Multivariate analysis was applied to test for differences in HRV between two different pacing states (Wilcoxon‘s one sample signed rank test). For univariate analysis Wilcoxon‘s signed rank test for paired observations for one and two sided alternatives was used.
RESULTS
The study population comprised 11 men and 9 women (mean (SD) age 59 (2) years), with a mean ejection fraction of 59 (14)%. Median HR during NSR1 was 69.8 beats/min (bpm). After the pacing manoeuvres (NSR2) median HR was 73.3 bpm (p = 0.6442). Comparison of NSR1 versus VAT pacing showed no significant differences in HR (69.8 v 72.3 bpm), SDNN (33.6 v 29 ms), or in RMSSD (19.5 v 17.9 ms), respectively (p = 0.3744). During AAI pacing SDNN decreased from 33.6 ms (NSR1) to 4.1 ms (AAI), as did RMSSD (19.5 ms during NSR1 to 4.1 ms during AAI, p < 0.0001). AAI pacing compared to VAT pacing yielded lower SDNN and RMSSD (4.1 v 31.4 ms for SDNN and 4.1 v 17.9 ms for RMSSD, p < 0.0001). To evaluate for measurement errors, DDD pacing was performed. SDNN was 1.2 ms and RMSSD was 1.3 ms.
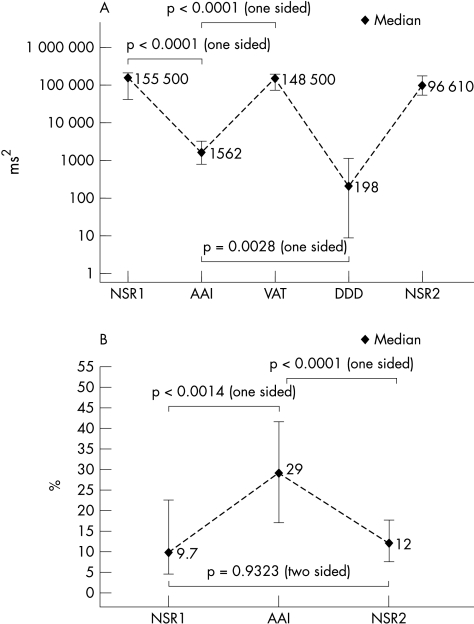
Figure 1A shows the total power during NSR1, AAI, VAT, DDD pacing, and NSR2. Total power drops from its median of 155 500 ms2 during the first sinus rhythm period to 1562 ms2 during AAI pacing (p < 0.0001). After AAI pacing is stopped and switched to VAT pacing the median of total power increases to 147 800 ms2 (p < 0.0001). The median of total power during DDD pacing is 199 ms2. Compared to AAI pacing, total power during DDD pacing is significantly smaller (p = 0.0028). No significant pairwise differences in total power between the periods NSR1, VAT pacing, and NSR2 were found.
Figure 1.
(A) Total power during NSR1, AAI, VAT, DDD pacing, and NSR2. Diamonds represent median values and vertical lines interquartile ranges. Values are presented on a logarithmic scale with the five periods on the x axis and total power in ms2 on the y axis. Total power is decreasing from NSR1 to AAI pacing (p < 0.0001). The difference for total power during AAI and DDD pacing is significant (p = 0.0028), which means that total power during AAI pacing is not just noise related. Furthermore, it indicates conduction variability in the AV node. (B) The relation of high frequency peak to total power between NSR1/NSR2 and AAI pacing differs significantly.
Figure 1B demonstrates the ratio of high frequency to total power during NSR1, AAI pacing, and NSR2. The median ratio is about 1:10 (range 1–45 %) during NSR1. During AAI pacing, the ratio rises to a median of 29% (p = 0.0014). The ratio reaches approximately the initial median value after discontinuation of atrial pacing. There is no significant difference in the ratio during NSR1 and NSR2 (p = 0.9323). Pre-existing conditions, such as coronary artery disease or depressed left ventricular function, showed no significant influence on HRV (three factorial multivariate analysis).
DISCUSSION
The major finding of this study is that HRV derived from RR intervals is mainly caused by variations in sinus node impulse formation. According to our results, AV nodal conduction contributes, as indicated by AAI pacing, but this contribution is very small and seems to be negligible under clinical conditions. Taylor and Eckberg3 attempted to estimate HRV during a short period (≤ 5 minutes) of transoesophageal pacing. Although atrial pacing was not stable and the influence of background noise could not be excluded, they also found a similar low SDNN of 4 (1) ms for patients lying in the supine position. Errors in measuring HRV during AAI pacing is unlikely, because median SDNN and RMSSD were at least three times higher than the control HRV during DDD pacing. We expected it to be equal to zero. However, quantified HRV during DDD pacing might represent a possible contribution from other structures or just noise level. This is emphasised by the significant difference in HRV between AAI and DDD pacing. Frequency domain analysis revealed an interesting change of the ratio of high frequency to total power during NSR1, AAI pacing, and NSR2. The relative contribution of high frequency peak to total power spectrum increases significantly to a median of 29% (p = 0.0014). This result suggests that cycle-to-cycle length variations in AV nodal conduction are significantly modulated by vagal tone. Our results indicate that the influence of autonomic tone over the AV node is very small.
In conclusion, HRV derived from RR intervals is caused predominantly by periodicity in sinus node impulse formation. Analysis of spectral parameters reveals a strong influence of the parasympathetic nervous system on AV node conduction variability.
Acknowledgments
We are grateful to Achim Domsel for assistance and technical support.
Abbreviations
AAI pacing, elimination of impulse formation in the sinus node
AV, atrioventricular
HR, heart rate
HRV, heart rate variability
NSR, normal sinus rhythm
RMSSD, square root of the mean of the squared successive differences in RR intervals
SDNN, standard deviation of all RR intervals
VAT pacing, AV sequential pacing
REFERENCES
- 1.European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- 2.Ori Z, Monir G, Weiss J, et al. Heart rate variability: frequency domain analysis. Cardiol Clin 1992;10:499–538. [PubMed] [Google Scholar]
- 3.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 1996;93:1527–32. [DOI] [PubMed] [Google Scholar]