Abstract
Objective: To compare the early and late outcome of medical and surgical treatment in patients with prosthetic valve endocarditis within a single unit.
Design: All patients with proven prosthetic valve endocarditis treated in one institution between 1989 and 1999 were studied.
Results: There were 66 patients (24 female, 42 male), mean (SD) age 57 (14) years. Of these, 28 were treated with antibiotics alone and 38 with a combination of antibiotics and surgery. The in-hospital mortality for the antibiotic group was 46% and for the surgical group, 24%. However, seven patients in the antibiotic group were considered too sick for curative treatment. The mortality in the remaining 21 medically treated patients (6/21; 29%) was not significantly different from that in the surgically treated patients (p = 0.15). Six patients in the medically treated group and one in the surgically treated group required late reoperation. Endocarditis recurred in three patients in the medically treated group, two of whom were treated surgically, and in one patient in the surgically treated group. Kaplan–Meier survival at 10 years was 28% in the medically treated group v 58% in the surgically treated group (p = 0.04). Freedom from endocarditis at five years was 60% in the surgically treated group and 65% in the medically treated group.
Conclusions: Prosthetic valve endocarditis is a serious condition with high early and late mortality, irrespective of the treatment employed. These data show that selected patients with prosthetic valve endocarditis can be successfully treated with antibiotics alone. If required, surgery in this difficult group of patients can provide satisfactory freedom from recurrent infection.
Keywords: prosthetic valve endocarditis, antibiotic treatment, surgical treatment
Prosthetic valve endocarditis remains a serious complication of heart valve surgery despite improvements in prophylaxis, diagnosis, and treatment. The traditional approach to the management of this condition has been early surgery, and superior results have been shown with surgical treatment compared with antibiotics alone.1–6 However, while early surgery is indicated in patients with haemodynamic compromise, there is evidence that in selected cases treatment with antibiotics alone provides equivalent results.7, 8
To compare the outcome of patients treated with antibiotics and surgery with patients treated with antibiotics alone, we performed a retrospective review of all cases of prosthetic valve endocarditis in our institution between 1989 and 1999.
METHODS
Between January 1989 and December 1999, 98 cases of prosthetic valve endocarditis were diagnosed and treated. From January 1989, patients treated for prosthetic valve endocarditis were prospectively identified and placed on a microbiological database and this forms the basis of this paper.
Patients were treated as having prosthetic valve endocarditis if they had clinical or biochemical evidence of sepsis plus three or more positive blood culture samples.
Clinical information was retrospectively obtained by a detailed review of clinical records and laboratory details. Mortality data were provided by the Trent regional birth and death registry service.
Definitions
For medically treated patients, prosthetic valve endocarditis was considered as proven if the case met Duke’s criteria9 or if necropsy confirmed prosthetic valve endocarditis was the cause of death. The diagnosis requires two major criteria or one major plus three of five minor criteria to be met. The major criteria are positive blood cultures of a typical microorganism, and evidence of endocardial involvement either from a positive echocardiogram or from new valvar regurgitation. The minor criteria are fever, vascular phenomena (arterial embolism, septic pulmonary infarct, mycotic aneurysm), immunological phenomena (glomerulonephritis, Osler’s nodes, Roth spots, Janeway lesions), a suggestive echocardiogram, and microbiological evidence (positive blood culture but not meeting the major criterion).
In patients who had surgery, if there was gross macroscopic evidence of infection at the time of surgery, if pathological lesions compatible with prosthetic valve endocarditis were seen at surgery, or if microorganisms were isolated from the explanted valve, the case was deemed to be proven prosthetic valve endocarditis.
Prosthetic valve endocarditis diagnosed within 60 days of valve insertion was defined as “early prosthetic valve endocarditis”.
Episodes were regarded as new rather than recurrent if they occurred more than six months after the last treated episode.
Treatment
For all patients in the medically treated group, treatment was started on admission to hospital and continued intravenously for at least two weeks, followed by oral treatment for at least six weeks. The initial choice of antibiotics was at the discretion of the clinician involved, but once the diagnosis was made, treatment was guided by a consultant microbiologist.
All patients in the surgically treated group were initially treated with antibiotics and had surgery during the same admission.
Statistics
Analysis was performed using the SPSS 10 statistical package. Proportions were compared with a χ2 test. Continuous parametric data were compared with a t test and non-parametric data with a Mann–Whitney test. Logistic regression was used to determine independent predictors of inpatient death and late survival. Long term survival was estimated using the Kaplan–Meier method and compared with a log rank test. A probability value of p < 0.05 was considered significant.
RESULTS
There were 2200 prosthetic heart valve replacements during the study period and 98 episodes treated as prosthetic valve endocarditis, giving an incidence of 4.5%. The rate of development of prosthetic valve endocarditis over the study period was 0.45% per year. Six patients had two episodes of endocarditis. These patients had their second episodes at 12, 13, 36, 37, 96, and 108 months after the first episode.
Of the 98 treated patients, 32 did not meet the criteria for proven prosthetic valve endocarditis and so were excluded from further analysis. The overall incidence of proven cases of prosthetic valve endocarditis was therefore 0.3% per year.
Clinical details
Of the 66 patients with proven prosthetic valve endocarditis, 28 were treated with antibiotics alone and 38 with antibiotics plus surgery. The clinical characteristics of the groups are shown in table 1. Seventeen of the 28 patients treated with antibiotics (61%) had positive blood cultures plus a diagnostic echocardiogram; 11 (39%) had positive blood cultures and at least three of the five minor criteria of endocarditis.
Table 1.
Clinical features
| Medical treatment (n=28) | Surgical treatment (n=38) | p Value | |
| Age (years) | 64 (12) | 51 (14) | 0.001 |
| Male patients | 16 (57%) | 26 (68%) | NS |
| Type of valve | |||
| Mechanical | 17 (60%) | 22 (58%) | NS |
| Bioprosthetic | 11 (40%) | 16 (42%) | NS |
| Location of PVE | |||
| Aortic | 10 (36%) | 27 (71%) | 0.02 |
| Mitral | 12 (43%) | 5 (13%) | NS |
| Both | 6 (21%) | 6 (16%) | NS |
| EPVE | 9 (32%) | 14 (37%) | NS |
Values are n (%) or mean (SD).
EPVE, early prosthetic valve endocarditis; PVE, prosthetic valve endocarditis
Antibiotic group
Among the 28 patients in this group there were 13 deaths, giving a mortality of 46%. Seven patients with multiorgan failure were not considered for curative treatment. All died within days of admission and had a necropsy examination. In all cases there were vegetations on the prosthetic valves, three had a paravalvar abscess, and one had partial dehiscence of a prosthetic aortic valve.
Among the remaining 21 patients considered for curative medical treatment, there were six deaths, giving a mortality of 29%.
Six patients initially treated with antibiotics subsequently had surgery. Two patients had recurrent prosthetic valve endocarditis at 12 and 36 months, respectively. The other four had surgery for a paravalvar leak at 6, 6, 36, and 108 months, with no evidence of sepsis at the operation. One patient had recurrent prosthetic valve endocarditis and was treated medically.
Surgical group
Mean (SD) time to surgery after diagnosis was 22 (15) days. All cases had evidence of prosthetic valve endocarditis at operation. The operative mortality was 24% (9/38).
The indications for surgery were one or more of the following: persistent sepsis despite appropriate antibiotic treatment (15); heart failure (10); echocardiographic findings, for example a paravalvar leak, annular abscess, or valve dehiscence (10); embolic phenomena (cerebrovascular accident (1); femoral artery septic embolus (1)); and complete heart block (1).
A valve replacement was the most common procedure. Four patients underwent extensive annular debridement requiring reconstruction with pericardium, two had aortic root replacements, and one required repair of an aortic to right atrial fistula.
One patient had redo surgery for a recurrent infection five months after the second operation. She died 13 months after this from heart failure, with no evidence of recurrent prosthetic valve endocarditis or valve dysfunction.
Organisms
The organisms involved are shown in table 2. There were more cases of coagulase negative staphylococcal prosthetic valve endocarditis in the surgically treated group than in the medical group (p = 0.003). The group “other” contains Mycoplasma pneumoniae (1), Coxiella burnetti (1), Listeria monocytogenes (1), Candida albicans (4), and Aspergillus species (1). In a logistic regression the only predictor of in-hospital death was infection with Staphyloccocus aureus (p = 0.01). The type of valve, the position of infected prosthesis, and early prosthetic valve endocarditis were not significant factors.
Table 2.
Microbiological data
| Medical treatment (n=28) | Surgical treatment (n=38) | p Value | |
| Staphylococcus species | 11 (40%) | 18 (47%) | NS |
| S aureus | 10 | 7 | NS |
| Coagulase negative | 1 | 11 | 0.003 |
| Streptococcus species | 6 (21%) | 10 (26%) | NS |
| Gram negative rods | 1 (4%) | 2 (5%) | NS |
| Other | 6 (21%) | 2 (5%) | 0.04 |
| Enterococcus | 4 (14%) | 6 (16%) | NS |
Echocardiographic findings
Seventeen patients in the medically treated group and all those in the surgically treated group had echocardiographic evidence of prosthetic valve endocarditis. These findings are summarised in table 3. The term “new regurgitation” described patients with moderate or severe regurgitation not noted previously.
Table 3.
Echocardiographic findings*
| Medical treatment (n=28) | Surgical treatment (n=38) | |
| Vegetations | 4 (14%) | 10 (26%) |
| Paravalvar leak | 3 (11%) | 4 (11%) |
| Annular abscess | 2 (7%) | 6 (16%) |
| Aortoventricular dehiscence | 0 | 2 (5%) |
| New valve regurgitation | 14 (37%) | 21 (55%) |
*More than one finding was present in some patients.
Long term results
The closing interval was six months. Follow up was 90% complete. Six patients had left the area and were not traceable.
Endocarditis recurred in three patients in the medically treated, two of whom were treated surgically, and in one patient in the surgical group. Six patients in the antibiotic group and one in the surgical group had another operation after the first episode.
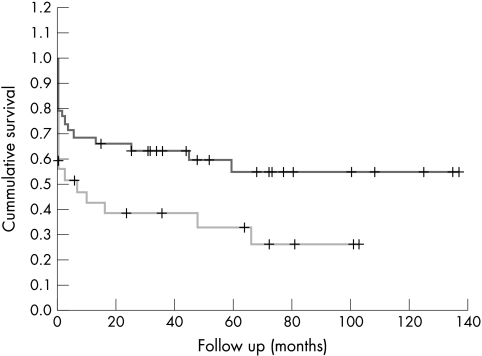
Ten year survival in the two groups, estimated by Kaplan–Meier analysis, was 28% in the antibiotic group v 58% in the surgical group (p = 0.04) (fig 1). In a logistic regression, the only predictor of late death from endocarditis was early prosthetic valve endocarditis (p = 0.05).
Figure 1.
Long term survival after surgical (upper graph) or medical (lower graph) treatment for prosthetic valve endocarditis.
The mode of treatment, organism, and position of infected prosthesis were not significant factors in predicting the outcome.
Freedom from endocarditis at five years was 60% in the surgically treated group and 65% in the medically treated group (p = 0.1).
DISCUSSION
Antibiotics have a major role to play in the treatment of prosthetic valve endocarditis. However, it is still unclear which subgroup of patients can be treated with antibiotics alone with a reasonable chance of cure. One major problem in assessing the efficacy of antibiotic treatment is that previous studies contained a spectrum of patients in whom the diagnosis of prosthetic valve endocarditis was not proven. It was therefore uncertain that patients who were successfully treated with antibiotics alone had endocarditis at all. For example in the study by Yu and colleagues,1 only 42 of the 74 patients had definite endocarditis, and in Kuyvenhoven’s study,10 only 39 of 70 episodes of prosthetic valve endocarditis were proven. To allow valid interpretation of the data in our study we included only patients in whom the diagnosis of prosthetic valve endocarditis was proven. To achieve this, almost one third of the patients treated as having this condition were excluded.
The second criticism of previous studies is the small number of patients reported. For example, in Trunninger’s study,7 there were only 10 patients in the antibiotic group. Compared with other studies which use stringent criteria to prove the diagnosis of prosthetic valve endocarditis, our study is relatively large.
Despite the relative imprecision of the diagnosis of prosthetic valve endocarditis in previous studies, mortality rates with antibiotic treatment alone have traditionally been high: 70% in the study by Ivert et al,11 53% in the one by Yu et al,1 and 26% in that by Kuyvenhoven et al.10 The mortality rate of 29% in our study is therefore comparable. The mortality of the patients who had surgery was again comparable with the findings from other studies: 23% in the study by Yu et al,1 30% in that by Kuyvenhoven et al,10 and 48% in the one by Grover et al.12
Our study shows that some patients with prosthetic valve endocarditis can be treated with antibiotics alone. The patients most likely to benefit from this approach are those who remain clinically stable or show improvement on antibiotic treatment. Echocardiographic features such as the absence of an abscess, the absence of valvar dysfunction or dehiscence, and infection limited to the leaflet of the bioprosthesis may indicate successful treatment with antibiotics alone. Prosthetic valve endocarditis caused by less virulent organisms such as coagulase negative Staphylococcus spp and viridans Streptococcus species may also be successfully treated with antibiotics alone. Finally, this approach may be used in patients in whom surgery is contraindicated for some other reason, or in cases where the patient refuses to consent to an operation.
The high rate of subsequent valve failure in patients not treated with surgery is an argument for surgical treatment of prosthetic valve endocarditis. Calderwood showed that patients not treated surgically during their initial hospital admission are at high risk of progressive prosthesis dysfunction and require careful follow up.2 Our findings support this. Of the 21 patients initially treated with antibiotics alone, 29% (6/21) required reoperation at a later date for valve failure. The fact that there were no deaths in this group suggests that initial medical management with subsequent surgery if indicated is a reasonable strategy.
Limitations
Our study has several shortcomings. A prospective randomised controlled trial remains the best way to evaluate the effectiveness of different strategies in the management of prosthetic valve endocarditis. Such a trial would take several years in order to recruit a sufficient number of patients in a single centre, or may have to involve multiple centres, leading to a non-homogeneous group of patients and management protocols. Thus a retrospective review is a reasonable mechanism for attempting to answer this question.
Clearly retrospective studies are hampered by lack of randomisation and therefore bias in the selection of treatment methods. For example “less sick” patients may have been more likely to have been treated medically. In this particular study, surgically treated patients were younger, 54 v 61 years old, and may have had less comorbidity. This may explain the improved long term survival of the surgical group.
In addition, there was no formal policy in the unit during the period studied. However, the overall strategy was to manage patients medically initially, and to offer surgery to those with complications or in whom medical treatment had been unsuccessful, rather than to operate simply because a diagnosis of prosthetic valve endocarditis had been made.
Finally, we acknowledge the possibility that medically treated patients may have had better results if they had had surgery. Again this issue can only be addressed in a large prospective randomised trial which would present a considerable challenge.
Conclusions
Our study shows that selected patients with prosthetic valve endocarditis can be treated successfully with antibiotics alone. Subsequent operations may be needed in the medically treated group. There is a possibility that patients given medical treatment might have done even better with surgery. Nevertheless, we believe that a strategy of reserving surgery for selected patients is justified by these results.
Acknowledgments
We thank the cardiothoracic surgeons and the cardiologist at the Northern General Hospital for their help in collecting these data.
REFERENCES
- 1.Yu VL, Fang GD, Keys TF, et al. Prosthetic valve endocarditis: superiority of surgical valve replacement versus medical therapy only. Ann Thorac Surg 1994;58:1073–7. [DOI] [PubMed] [Google Scholar]
- 2.Calderwood SB, Swinsky LA, Karchmer AW, et al. Prosthetic valve endocarditis: analysis of factors affecting outcome of therapy. J Thorac Cardiovasc Surg 1986;92:776–8. [PubMed] [Google Scholar]
- 3.Saffle JR, Gardner P, Schoenbaum SC, et al. Prosthetic valve endocarditis: the case for prompt valve replacement. J Thorac Cardiovasc Surg 1977;3:416–20. [PubMed] [Google Scholar]
- 4.Riccicciioli C, Chastre J, Lecompte Y, et al. Prosthetic valve endocarditis: the case for prompt surgical management. J Thorac Cardiovasc Surg 1986;92:784–9. [PubMed] [Google Scholar]
- 5.Lytle BW, Taylor PC, Sapp SK, et al. Surgical treatment of prosthetic valve endocarditis. J Thorac Cardiovasc Surg 1996;111:198–210. [DOI] [PubMed] [Google Scholar]
- 6.Farina G, Vitale N, Piaza L, et al. Long term results of surgery for prosthetic valve endocarditis. J Heart Valve Dis 1994;2:165–71. [PubMed] [Google Scholar]
- 7.Trunninger K, Attenhofer CH, Seifert B, et al. Long term follow up of prosthetic valve endocarditis: what characteristics identify patients who were treated successfully with antibiotics alone. Heart 1999;82:714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karchmer AW, Dismuke WE, Buckley MJ, et al. Late prosthetic valve endocarditis clinical features influencing therapy. Am J Med 1978;64:199–206. [DOI] [PubMed] [Google Scholar]
- 9.Durack DT, Lukes AS, Bright DK. New criteria for the diagnosis of infective endocarditis: utilization of specific echcardiographic findings. Am J Med 1994;96:200–9. [DOI] [PubMed] [Google Scholar]
- 10.Kuyvenhoven P, Rijk-Zwikker GL, Hermans J, et al. Prosthetic valve endocarditis: analysis of risk factors for mortality. Eur J Cardiothorac Surg 1994;8:420–4. [DOI] [PubMed] [Google Scholar]
- 11.Ivert TS, Dismukes WE, Cobbs CG, et al. Prosthetic valve endocarditis. Circulation 1984;69:223–32. [DOI] [PubMed] [Google Scholar]
- 12.Grover FL, Cohen DJ, Oprian C, et al. Determinants of the occurrence of and survival from prosthetic valve endocarditis. J Thorac Cardiovasc Surg 1994;108:207–13. [PubMed] [Google Scholar]