The last decade has seen a revolution in the technique of contrast echocardiography, with detailed understanding of the interaction between microscopic air bubbles and ultrasound leading to rapid development in both contrast and imaging technology. Several transpulmonary contrast agents have been developed, capable of crossing the pulmonary vascular bed and hence achieving left heart opacification following intravenous injection. Combined with major developments in cardiac ultrasound equipment, left heart contrast echocardiography has moved from research into mainstream clinical echocardiography and we stand on the threshold of myocardial contrast echocardiography as a tool for routine clinical use in the assessment of the patient with ischaemic heart disease. Optimal use of these techniques requires detailed understanding of the agents used, mode of administration, and optimum machine settings. Basic knowledge of the physics of ultrasound is now crucial if the potential for contrast echocardiography is to be realised in clinical practice.
▸ CONTRAST ECHOCARDIOGRAPHY: PHYSICAL PRINCIPLES
Blood appears black on conventional two dimensional echocardiography, not because blood produces no echo, but because the ultrasound scattered by red blood cells at conventional imaging frequencies is very weak—several thousand times weaker than myocardium—and so lies below the displayed dynamic range. Contrast ultrasound results principally from the scattering of incident ultrasound at a gas/liquid interface, increasing the strength of returning signal. However, the bubble/ultrasound interaction is complex and its nature has only recently been fully elucidated. Understanding this interaction is key to performing, understanding, and interpreting a contrast echo study.
When insonated, gas bubbles pulsate, with compression at the peak of the ultrasound wave and expansion at the nadir. Electron microscopy studies have eloquently demonstrated the extent of this volume pulsation with bubble radius changing by a factor of 20 or more. In an ultrasound beam with a frequency of 3 MHz, this will result in bubble oscillation three million times per second. With this movement, sound is generated and, combined with that of thousands of other bubbles, results in the scattered echo from the contrast agent. Characterising this echo, such that it can be differentiated from that of tissue, improves the sensitivity of contrast ultrasound and is the basis for new contrast specific imaging modes detailed below.
Unlike solid tissue, gas bubbles have acoustic properties that vary with the strength of the insonating signal. With increasing power, insonation of gas bubbles can result in linear oscillation, non-linear oscillation or bubble destruction (scintillation).1 Linear oscillation will augment the echo signal from the blood pool, and was the behaviour originally envisaged as the major source of contrast. However, in reality, the pressure generated by conventional ultrasound equipment greatly exceeds that required to generate linear oscillation, with non-linear oscillation and bubble destruction the result. This in part underlies the transient nature of the contrast effect with conventional and first generation contrast agents.
Contrast bubbles oscillating in an ultrasound beam are several million times more effective at scattering sound than red blood cells and as a result greatly enhance the blood pool signal. In Doppler ultrasound this results in a dramatic increase in signal strength, in the order of 10–20 dB. This property has been utilised for many years using agitated saline to augment right heart Doppler signals and the initial clinical indication for left heart echo contrast agents was to enhance Doppler examination. Intravenous Levovist was shown to result in dramatic enhancement of both spectral and colour Doppler, capable of increasing the technical success rate in patients with suboptimal Doppler studies.2 This modality is useful in the assessment of aortic stenosis, with contrast enhancing the Doppler envelope (fig 1) and can enable pulmonary vein flow analysis to be performed from the transthoracic apical four chamber view in most patients.2 The ability of contrast to enhance Doppler signal strength led to the development of power Doppler imaging (also known as Doppler energy imaging) in which the amplitude of the Doppler signal, rather than velocity, is displayed. Power Doppler is more sensitive to the effect of contrast but is much more prone to interference from clutter. This downside is largely overcome with the addition of harmonic imaging.
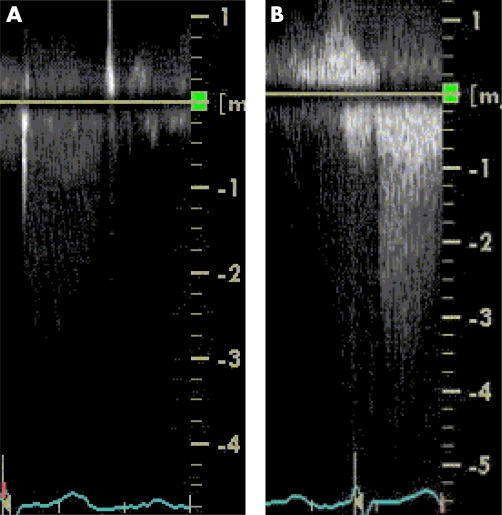
Figure 1.
Spectral Doppler enhancement. (A) Spectral Doppler of flow across the aortic valve in a patient with suboptimal imaging from the apical window resulting in poor quality Doppler envelope. The maximum velocity recorded was 2.6 m/s. (B) Intravenous injection of 0.5 ml of SonoVue resulted in dramatic enhancement of the Doppler signal, enabling more accurate quantification of aortic stenosis gradient. The maximum velocity recorded was 4.1 m/s.
Insonated gas bubbles display the physical property of resonance—a frequency of oscillation at which the absorption and scattering of ultrasound is particularly efficient. It appears a remarkable and fortuitous coincidence that gas bubbles of a size required to cross the pulmonary capillary vascular bed (1–5 μm) resonate in a frequency range of 1.5–7 MHz, precisely that used in diagnostic ultrasound. Insonation of gas bubbles at their resonant frequency results in non-linear oscillation of the bubble, whereby the alternate expansion and contraction of the bubble is not equal. This results in the generation of harmonics—ultrasound produced at a frequency which is a multiple of the insonating frequency. Recognition of this property of contrast media led to the development of harmonic imaging.3 With the receiver tuned to receive double the transmit frequency, an image is generated predominantly from the first harmonic signal, greatly improving the signal-to-noise ratio. Although initially developed as an aid to contrast echo, tissue also generates harmonics and the ability to enhance conventional grey scale imaging was rapidly appreciated. Harmonic imaging is now a standard feature on most ultrasound machines marketed today. Current developments to enhance sensitivity to contrast agents still further are concentrating on the subharmonic (1/2 insonation f) and ultraharmonic (1.5 f) signals, with a “signature” more specific to contrast agents. By imaging contrast agent and not tissue, tissue perfusion can potentially be identified. The harmonic response is dependent upon the physical characteristics of the agent (both size and mechanical properties), the incident pressure of the ultrasound field, and the frequency. Thus, optimal contrast imaging must be set up for the agent and equipment in use.
Harmonic imaging greatly improves sensitivity to contrast and enables excellent left ventricular cavity opacification (fig 2), but resolution is insufficient for tissue perfusion imaging. Pulse inversion and power modulation imaging have been developed to improve the differentiation of contrast from tissue still further.4 By alternating the phase or amplitude of sequential pulses of ultrasound sent along the same scan line and summing the received signal, ultrasound pulses returning from tissue cancel each other out, while that from contrast, producing greater harmonics, is enhanced. These methods are so sensitive to contrast that weaker echoes from bubbles insonated at very low intensity can be readily imaged, resulting in oscillation without bubble destruction, prolonging the contrast effect and enabling real time myocardial perfusion imaging.
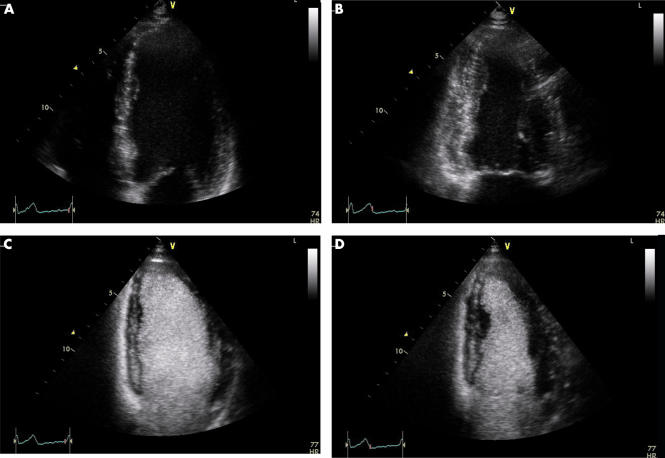
Figure 2.
Left ventricular opacification. Apical four chamber view, end diastolic (A) and end systolic (B) frames, in a patient with recent myocardial infarction referred for assessment of left ventricular systolic function. Images acquired utilising tissue harmonic imaging at frequency of 3.8/1.9 MHz and a mechanical index of 1.0. Lateral wall endocardium was not clearly defined making accurate measurement of left ventricular volumes difficult. Apical four chamber view, end diastolic (C) and end systolic (D) frames, imaged after intravenous bolus injection of 0.5 ml of SonoVue. The mechanical index has been reduced to 0.5 but all other parameters are unchanged. The entire endocardial border is now clearly defined and systolic thickening of entire lateral wall appreciated.
Contrast imaging requires ultrasound machine settings to be optimised for the modality used. Generally, this requires variation in the system power output, indicated on clinical systems as the mechanical index (MI). This is an estimate of the peak negative pressure within insonated tissue defined as the peak negative pressure divided by the square root of the ultrasound frequency. This is clearly dependent on both ultrasound beam properties and tissue characteristics but the latter is assumed to be standard across all patients. Display of the MI was made mandatory in the USA as a safety measure, to enable an estimate of the tissue effects of ultrasound exposure to be made. As this also reflects the mechanical effect of ultrasound on a contrast bubble, this has proved useful in developing machine settings for contrast ultrasound. It is relatively imprecise and not directly comparable from machine to machine, but is nonetheless one of the most important parameters to set correctly in a contrast echo study. Standard clinical echocardiography imaging utilises an MI of around 1.0, but a lower setting, around 0.5, is usually optimal for left ventricular opacification. To achieve myocardial perfusion imaging the extremes of power output are utilised: high MI (> 1.2) is used to achieve bubble destruction in power Doppler imaging, and ultra low MI (< 0.1) required to induce linear oscillation of microbubbles required for real time myocardial perfusion imaging.
CONTRAST AGENTS FOR ULTRASOUND
Initially contrast echocardiography utilised free air in solution but these large, unstable bubbles were not capable of crossing the pulmonary vascular capillary bed, allowing right heart contrast effects only. The first agents capable of left heart contrast after intravenous injection (first generation agents) were air bubbles stabilised by encapsulation (Albunex) or by adherence to microparticles (Levovist). Replacing air with a low solubility fluorocarbon gas stabilises bubbles still further (second generation agents—for example, Optison, SonoVue), greatly increasing the duration of the contrast effect.5 Third generation agents—not yet commercially available—will use polymer shell and low solubility gas and should produce much more reproducible acoustic properties.
PERFORMING A CONTRAST STUDY
Bubbles in contrast agents are delicate and prone to destruction by physical pressure. Performing a contrast study requires meticulous attention to the preparation and administration of the contrast agent to optimise the desired effect. The agent should be prepared immediately before injection and vents used when withdrawing the agent into the syringe. Bubbles tend to float towards the surface and the contrast vial or syringe should be gently agitated each time fresh contrast administration is required. Injection through a small lumen catheter increases bubble destruction—a 20 G or greater cannula should be used. Very small volumes of contrast are needed using second generation agents (< 1 ml) and a flush is required. This is best done using a three way tap, with contrast injected along the direct path to minimise bubble destruction, and saline flush injected into the right angle bend. For myocardial perfusion work, infusion produces more reproducible results with the potential for quantification6 but brings its own problems. Bubbles in agents currently available are buoyant and will tend to rise to the surface of the syringe. Purpose designed infusion pumps which agitate contrast continuously are in development but not yet widely available.
CONVENTIONAL CONTRAST ECHOCARDIOGRAPHY
Conventional contrast echocardiography is performed predominantly with agitated saline. This is most readily achieved by hand agitation of saline between two 10 ml syringes connected to a three way tap. Luer lock syringes are recommended to avoid accidental spraying of the echo lab with saline and is particularly important for safety reasons if a blood/saline mixture is used (this achieves greater intensity and more prolonged effect but not sufficient to justify the risk of contamination). Approximately 5 ml of saline and 0.2 ml of air should be rapidly injected from one syringe to the other until it appears opaque but with no large visible air bubble. Contrast should be injected immediately after preparation. Air bubbles produced by hand agitation are too large to cross the pulmonary vascular bed and so predominantly aid visualisation of the right heart. Thus, any significant contrast effect in the left heart is likely to be the result of an intracardiac shunt.
The main clinical applications of this technique are detailed in table 1. By far the most common indication in adult cardiology is the detection of patent foramen ovale (PFO) in patients in whom paradoxical embolism is suspected. Use of harmonic imaging and a relatively low MI will enhance the contrast effect of agitated saline, with the result that most clinically important intracardiac shunts and PFO can be detected during transthoracic echocardiography, with sensitivity greater than that of a transoesophageal examination.7 Indeed, mode of injection may be of more relevance than imaging modality, with injection into the femoral vein demonstrating a large intracardiac shunt in some patients with negative study after antecubital vein injection.7 A PFO is diagnosed if more than three microbubbles pass from right to left atrium within three cardiac cycles of right atrial opacification. Crude quantification is possible with a small shunt defined as 3–10 bubbles, a medium shunt 10–20, and a large shunt > 20 bubbles. An initial study should be performed during normal respiration, when the normal reversal of atrial pressure gradient in early systole may be sufficient to allow shunting if a large defect is present. If negative the study should be repeated during provocative manoeuvres that transiently raise right atrial pressure above left—Valsalva’s manoeuvre, coughing or firm abdominal pressure (useful if a patient has required heavy sedation for transoesophageal study). Valsalva’s manoeuvre is the most effective; the patient should be asked to strain at the time of injection and release breath as the right atrium begins to opacify. If successfully performed, the atrial septum can be seen to bow transiently from right to left. PFO is common, being present in 25–30% of the normal population and as many as 74% of young patients with stroke, and its management remains contentious.8
Table 1.
Indications for conventional contrast echocardiography
| Indication | Evaluation |
| Cardiac shunt (ASD, VSD, etc) | Negative contrast effect: “hole” in opacified RA or RV |
| Easy passage of contrast to LA or LV | |
| Extracardiac shunt: PDA | Visualisation of contrast in aorta and peripheral circulation without left heart effect |
| –pulmonary AV fistula | Appearance of contrast in LA via pulmonary veins |
| Patent foramen ovale | Contrast in LA immediately after visualisation in RA |
| Structure identification (persistent left SVC) | Visualisation of contrast in enlarged coronary sinus and RA after injection into left (but not right) antecubital vein |
| Evaluation of TR | Enhanced colour and spectral Doppler |
| Evaluation of complex congenital heart disease | Combination of above |
ASD, atrial septal defect, AV, arteriovenous; LA, left atrium; LV, left ventricle; PDA, patent ductus arteriosus; RA, right atrium; RV right ventricle; SVC, superior vena cava; TR, tricuspid regurgitation; VSD, ventricular septal defect.
LEFT VENTRICULAR OPACIFICATION
Assessment of left ventricular (LV) systolic function is the most common indication for echocardiography. Accurate assessment and quantification is dependent on visualising the entire endocardium in cross section. Tissue harmonic imaging has greatly improved image quality and reduced the number of non-diagnostic studies, but between 5–10% of echo examinations are suboptimal. These problems are amplified still further when performing stress echocardiography, where recognition of regional wall motion defects is crucial but frequently hampered by reduced image quality resulting from rapid heart rate, increased cardiac translocation, and respiratory interference. Contrast opacification of the left ventricular cavity enhances endocardial border definition (fig 2) and has been shown to increase diagnostic accuracy in suboptimal studies at rest9 and during stress.10 This is the major clinical use of left heart contrast echocardiography at present (table 2). Most centres performing stress echocardiography will now routinely use contrast in the majority of studies. Contrast echocardiography has been shown to be particularly useful in the assessment of LV function in patients ventilated in intensive care, reducing the time required to obtain diagnostic information and obviating the need for transoesophageal echocardiography.11 It is not a panacea, however. Contrast will not enable visualisation of the left ventricle when baseline images are totally inadequate. It is of most use when between a quarter and a half of endocardial segments are not well visualised. While apical imaging is greatly enhanced, parasternal views may deteriorate, at least initially, with contrast in the right ventricle attenuating visualisation of the left. For this reason, apical views should routinely be obtained first in any contrast study.
Table 2.
Indications for left ventricular (LV) contrast opacification
| Use | Indication |
| Accurate assessment of LV systolic function | Suboptimal imaging—for example, ventilated patient |
| Automated edge detection | |
| Recognition of regional wall motion abnormalities | Stress echocardiography |
| Confirm/exclude LV thrombus | Suspected apical filling defect |
| Delineate LV structure | Pseudoaneurysm |
| Apical hypertrophic cardiomyopathy | |
| Non-compaction of left ventricle | |
| Intracardiac mass (vascularity) |
LV opacification is also used to delineate LV anatomy, particularly apically, confirming pseudoaneurysm, apical hypertrophy or ventricular non-compaction and demonstrating filling defects, typically apical thrombus.
LV opacification studies should routinely utilise harmonic B mode imaging with the MI set to approximately 0.5. Compression is optimally set in the medium to high range and focus should be set at the level of the mitral valve or below. Intravenous bolus injection is the simplest method of contrast administration but infusion may be utilised, particularly during stress studies. If the MI is too high or focus wrongly set, excessive destruction of contrast in the near field will result in apical swirling. If too much contrast is used, visualisation of basal segments may be attenuated. As bubble destruction occurs during imaging, this will spontaneously resolve, but a lower dose should be used for subsequent imaging. In some studies, lateral wall dropout caused by rib artefact can prevent imaging of entire endocardium. This can usually be overcome by repositioning the image with the lateral wall more central.
Power Doppler imaging of the left ventricular cavity is more sensitive to the presence of contrast and can be tried if harmonic B mode provides inadequate images, but is limited by a reduced frame rate (5–10 frames/second), insufficient for accurate wall motion analysis, with greater tendency to wall motion artefacts. In the most difficult to image patient, triggering of the power Doppler image to obtain frames at end systole and end diastole only can greatly improve sensitivity and at least allow an estimate of LV volume and ejection fraction to be made. This is potentially an ideal method to utilise with automated contour detection algorithms for calculation of LV ejection fraction.
MYOCARDIAL PERFUSION IMAGING
Myocardial contrast echocardiography (MCE)—the imaging of a contrast agent within the myocardial capillary vascular bed—offers the potential of reproducible, real time, non-invasive assessment of myocardial perfusion during rest and stress, at the bedside and in the cardiac catheterisation laboratory. As such, it has long been the holy grail of echocardiographic assessment of the ischaemic patient, with its potential amply demonstrated by animal studies and work in man utilising intracoronary injection of sonicated contrast media.12
At present, MCE remains difficult and suboptimal imaging prohibits routine use. While LV cavity opacification with contrast can make a difficult study diagnostic, only those with high quality baseline B mode images are suitable for MCE with current equipment. Intravenous injection of contrast results in a very low concentration of bubbles in the myocardium, with only 5–10% of cardiac output entering the coronary circulation. As more than 90% of intramyocardial blood volume is within the capillary compartment, contrast bubbles are imaged at low velocity with slow replenishment following bubble destruction. Dissolution and destruction of microbubbles by both intramural pressure and ultrasound exposure further limits any contrast effect. Thus, contrast specific imaging modalities must be used. Broadly, there are two approaches used to overcome the problems inherent in myocardial contrast perfusion imaging : intermittent imaging and pulse inversion or power modulation imaging.
Intermittent imaging reduces exposure of microbubbles to ultrasound, with one frame imaged every 1–8 cardiac cycles, usually triggered at end systole on the ECG. Intermittent power Doppler imaging was the first modality to convincingly demonstrate myocardial perfusion after intravenous injection.13 High MI is utilised to cause bubble scintillation, with bubble destruction causing disruption of the ultrasound beam and loss of correlation of the Doppler processing algorithm. As no signal is detected pre-contrast and a high signal detected from stationary or slow moving bubbles, the entire myocardial circulation can be detected. Intermittent imaging enables microvascular replenishment before the next imaging cycle. By determining the number of cardiac cycles necessary to allow replenishment of the myocardial vascular bed, crude quantification of myocardial blood flow is achieved.14 This method remains the most sensitive currently available tool in clinical practice, particularly suited to imaging of patients with myocardial infarction where the question is one of flow or no-flow (fig 3). MCE appears to correlate well with coronary flow reserve and predict recovery in systolic function after reperfusion therapy in this setting.14,15 However, it is technically challenging, with loss of real time imaging making it more difficult to maintain the imaging plane, and wall motion artefacts occasionally limiting visualisation.
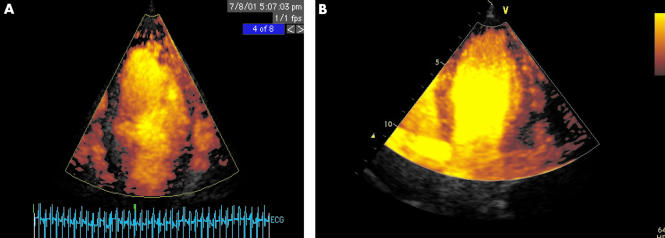
Figure 3.
Myocardial contrast echocardiography utilising intermittent power Doppler imaging triggered at end systole, after injection of a 0.5 ml bolus of Optison. Apical 4 chamber views. (A) Patient with recent anteroseptal myocardial infarction treated with thrombolysis but no clinical evidence of reperfusion. Absence of perfusion in septum and apex, compared to apical lateral segment, is readily appreciated. There is some dropout in the basal lateral segment caused by attenuation from apical contrast. (B) Patient with anteroseptal myocardial infarction treated successfully with thrombolysis. Normal contrast uptake in septum with minor apical defect only.
Real time MCE can be achieved using harmonic imaging of bubbles with very low MI, where tissue harmonics are minimal but contrast harmonics remain significant. Combined with pulse inversion or power modulation to amplify the contrast effect, myocardial blood flow can be visualised. The very low MI used means that bubble destruction from the incident ultrasound is minimal. If contrast is infused and a steady state achieved, this technique may enable quantification of myocardial blood flow which, combined with vasodilator stress, could enable detection of coronary stenoses in a manner similar to established nuclear cardiology techniques. Utilising harmonic power Doppler imaging and continuous infusion of Optison, concordance with adenosine single photon emission computed tomography (SPECT) of 81% has been demonstrated,16 with most disparity caused by false positive reporting of perfusion defects in the lateral wall with MCE.
Flash imaging, utilising a pulse of echo at high MI to destroy all microbubbles within the myocardium, combined with low MI real time MCE allows assessment of perfusion in real time (fig 4). This technique can be used to quantify rate of bubble replenishment in the myocardium.17 Pulsing interval plotted against the video intensity curve from myocardium during replenishment allows determination of the rate of rise of contrast within the myocardial vascular bed, β. After vasodilation with dipyridamole, β is significantly lower in the coronary vascular bed subtended by a stenosed coronary artery and initial clinical results suggest high concordance with nuclear imaging techniques.18
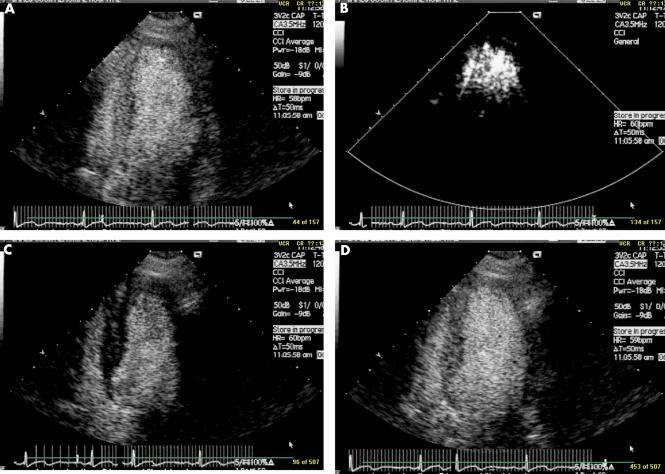
Figure 4.
Real time myocardial contrast echocardiography using pulse inversion and slow intravenous injection of 0.5 ml of Optison. Opacification of both left ventricular cavity and myocardium is seen in panel A. A pulse of high intensity ultrasound is delivered (B), destroying all contrast within myocardium. After this flash imaging, there is some residual contrast seen within the left ventricular cavity but myocardium appears completely black (C). Within three cardiac cycles, full replenishment of contrast within myocardium is seen (D). This allows crude quantification of myocardial blood flow. In normally perfused myocardium, full replenishment is apparent in less than four cardiac cycles. If replenishment occurs but takes between 4–10 cycles, coronary flow is reduced. If no replenishment has occurred after 10 cycles flow is absent. Plotting of the videointensity curve against pulsing interval can allow more accurate quantification of myocardial replenishment.
The enhanced spatial resolution of MCE compared to myocardial scintigraphy enables recognition of subendocardial defects, which may be a particularly sensitive marker of ischaemia. During stress echocardiography, it may be possible to detect abnormal flow related to coronary stenoses before regional wall motion abnormalities appear, enhancing diagnostic accuracy.19 Preliminary clinical results with other modalities appear to reinforce this.18
Infusion of contrast agent, necessary for quantitative real time MCE, is problematic. Improving contrast media stability and mode of delivery, combined with further refinement of echocardiographic equipment to improve image acquisition and interpretation of MCE data, is required to enable this technique to move fully from research to the clinical arena. Rate of development over the last decade and preliminary research work suggests that these goals will be met within the next 2–3 years.
CONCLUSIONS
Contrast echocardiography has evolved rapidly in the last decade, with major developments in both contrast media and ultrasound equipment. At the same time, understanding of the physical principles underlying the interaction of ultrasound and microbubbles has enhanced our ability to optimise the technique. This knowledge has improved the sensitivity of conventional contrast echo, used predominantly for the detection of intracardiac shunts, but more importantly resulted in routine use of contrast for left heart opacification to optimise assessment of left ventricular function during stress echocardiography and when imaging is suboptimal. Further refinement in technology has brought us to the threshold of MCE as a tool for the routine clinical assessment of myocardial perfusion. The future holds the promise of new contrast agents capable of imaging specific abnormalities in the vasculature, such as thrombi or damaged endothelium, while the next stage in this rapidly developing field is likely to move ultrasound from diagnostic technique to therapeutics. Preliminary work to use ultrasound mediated destruction of microbubbles conjugated with plasmid or adenoviral DNA as a means of delivering gene therapy to myocardium is well established.20
Contrast echocardiography: key points.
Contrast echocardiography utilises the interaction of microscopic gas bubbles with ultrasound to enhance recognition of blood pool and/or the blood/tissue interface
The principal use of conventional contrast echocardiography, utilising air bubbles produced by the agitation of saline, is the recognition of intracardiac shunting, particularly patent foramen ovale
Improved contrast agent technology combined with the introduction of harmonic imaging has increased sensitivity of the technique and expanded the indications of contrast echocardiography
Left ventricular opacification by left heart contrast can salvage a non-diagnostic study of left ventricular function, and is particularly useful during stress echocardiography
Recent developments in the technique of myocardial contrast echocardiography enables assessment of myocardial perfusion in both myocardial infarction and chronic ischaemic heart disease during intravenous infusion of left heart contrast. Quantification of myocardial blood flow by echocardiography is on the threshold of routine clinical use
REFERENCES
- 1.De Jong N, Frinking PJA, Bouakaz A, et al. Detection procedures of ultrasound contrast agents. Ultrasonics 2000;83:87–92. [DOI] [PubMed] [Google Scholar]
- 2.Von Bibra H, Sutherland G, Becher H, et al. Clinical evaluation of left heart Doppler contrast agent by a saccharide-based transpulmonary contrast agent. The Levovist cardiac working group.J Am Coll Cardiol 1995;25:500–8. ▸ Landmark article demonstrating successful use of a left heart contrast agent to enhance the Doppler signal in difficult to image patients. [DOI] [PubMed] [Google Scholar]
- 3.Burns PN, Powers JE, Simpson DH, et al. Harmonic imaging: principles and preliminary results. Clin Radiol 1996;51(suppl I):50–5.8605774 [Google Scholar]
- 4.Becher H, Burns PN. Contrast agents for echocardiography: principles and instrumentation. In: Becher H, Burns PN. Handbook of contrast echocardiography. LV function and myocardial perfusion. Berlin: Springer-Verlag, 2000:2–44. ▸ Small handbook with detailed explanation of principles behind contrast agents and imaging techniques, and detailed review of developing clinical use. Includes many practical tips on achieving optimal results for those new to the field. Available to download free of charge as pdf file at www.swchsc.on.ca/ EchoHandbook
- 5.De Jong N, Ten Cate FJ. New ultrasound contrast agents and technological innovations. Ultrasonics 1996;34:587–90. [DOI] [PubMed] [Google Scholar]
- 6.Wei K, Jayaweera AR, Firoozan S, et al. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion ? J Am Coll Cardiol 1998;32:252–60. ▸ Eloquent evaluation of two approaches for detection of coronary stenosis, with discussion of pros and cons of each. [DOI] [PubMed] [Google Scholar]
- 7.Kerut EK, Norfleet WT, Plotnick GT, et al. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001;38:613–23. ▸ Detailed review of pathophyiology of PFO, with emphasis on diagnosis and imaging. [DOI] [PubMed] [Google Scholar]
- 8.Alp N, Clarke N, Banning AP. How should patients with patent foramen ovale be managed ? Heart 2001;85:242–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Mansour HA, Mulvagh SL, Pumper GM, et al. Usefulness of harmonic imaging for left ventricular opacification and endocardial border delineation by Optison. Am J Cardiol 2000;85:795–9. [DOI] [PubMed] [Google Scholar]
- 10.Rainbird AJ, Mulvagh SL, Oh JK, et al. Contrast dobutamine stress echocardiography: clinical practice assessment in 300 consecutive patients. J Am Soc Echocardiogr 2001;14: 378–85. ▸ Demonstrates improvement in imaging of wall motion during stress with contrast, with no change in image quality between rest and peak. Includes technical learning points based on authors’ experience of the technique. [DOI] [PubMed]
- 11.Reilly JP, Tunick PA, Timmermans RJ. Contrast echocardiography clarifies uninterpretable wall motion in intensive care unit patients. J Am Coll Cardiol 2000;35:485–90. [DOI] [PubMed] [Google Scholar]
- 12.Kaul S. Myocardial contrast echocardiography. 15 years of research and development. Circulation 1997;96:3745–60. [DOI] [PubMed] [Google Scholar]
- 13.Porter TR, Xie F. Transient myocardial contrast after initial exposure to diagnostic ultrasound pressures with minute doses of intravenously injected microbubbles: demonstration and potential mechanisms. Circulation 1995;92:2391–5. [DOI] [PubMed] [Google Scholar]
- 14.Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol 2001;38:19–25. ▸ Used triggered imaging to obtain perfusion images in patients with recent myocardial infarction and demonstrated ability of technique to identify viable myocardium. [DOI] [PubMed] [Google Scholar]
- 15.Lepper W, Hoffman R, Kamp O, et al. Assessment of myocardial perfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angiography in patients with acute myocardial infarction. Circulation 2000;101:2368–74. ▸ Used MCE to define area of “no reflow” and correlated findings with coronary flow reserve measured by intracoronary Doppler flow wire. MCE correlated with coronary flow reserve and predicted functional recovery at four weeks. [DOI] [PubMed] [Google Scholar]
- 16.Heinle SK, Noblin J, Goree-Best P, et al. Assessment of myocardial perfusion by harmonic power Doppler imaging at rest and during adenosine stress. Comparison with 99-Tc sestamibi SPECT imaging. Circulation 2000;102:55–60. ▸ Demonstrates both potential strength and weakness of MCE as an alternative to radionuclide perfusion imaging. [DOI] [PubMed] [Google Scholar]
- 17.Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473–83. [DOI] [PubMed] [Google Scholar]
- 18.Gunda M, Mulvagh SL. Recent advances in myocardial contrast echocardiography. Curr Opin Cardiol 2001;16:231–9. ▸ Review article detailing recent clinical experience and results from published literature and work in progress. [DOI] [PubMed] [Google Scholar]
- 19.Porter TR, Xie F, Silver M, et al. Real-time perfusion imaging with low mechanical index pulse inversion Doppler imaging. J Am Coll Cardiol 2001;37:748–53. ▸ Perfusion imaging performed during stress echocardiography and findings correlated with quantitative coronary angiography. MCE proved superior to wall motion analysis in detection of significant stenoses. [DOI] [PubMed] [Google Scholar]
- 20.Vannan M, McCreery T, Li P, et al. Ultrasound-mediated transfection of canine myocardium by intravenous administration of cationic microbubble-linked plasmid DNA. J Am Soc Echocardiogr 2002;15:214–18. [DOI] [PubMed] [Google Scholar]