Abstract
Background: Electron beam computed tomography (EBCT) and multislice computed tomography (MSCT) are both suitable for non-invasive identification of coronary stenoses.
Objective: To compare intravenous coronary EBCT angiography (EBCTA) and MSCT angiography (MSCTA) with regard to image quality and diagnostic accuracy.
Methods: EBCTA was done using an Imatron C-150 XP scanner in 101 patients following a standard protocol (slice thickness 3 mm, overlap 1 mm, acquisition time 100 ms, prospective ECG trigger). For MSCTA in a different set of 91 patients (using a Siemens Somatom Plus4VZ scanner), the whole volume of the heart was covered in a spiral technique by four simultaneous detector rows. Using retrospective ECG gating, the raw data were reconstructed in (mean (SD)) 215 (12) axial slices acquired in diastole (slice thickness 1.25 mm, overlap 0.5 mm, acquisition time 250 ms/slice).
Results: With EBCTA, 76% of predetermined coronary segments in a nine segment model could be assessed with diagnostic image quality, and with MSCTA, 82%. A low contrast to noise ratio with EBCTA, and the presence of motion artefacts with MSCTA were the main reasons for inadequate image quality. Using conventional angiography as the gold standard, 77% of stenoses of > 50% could be identified correctly with EBCTA and 82% with MSCTA. Significant stenoses were correctly ruled out in 93% of segments with EBCTA, and in 96% of segments with MSCTA. The average contrast to noise ratio was higher with MSCTA than with EBCTA (9.4 v 6.5; p < 0.001).
Conclusions: EBCTA and MSCTA show similarly high levels of accuracy for determining and ruling out significant coronary artery stenoses. MSCTA is capable of providing good image quality in more coronary segments than EBCTA because of its better contrast to noise ratio and higher spatial resolution. Motion artefacts seen at heart rates of > 75 beats/min and a higher radiation exposure are the main limitations of MSCTA.
Keywords: electron beam tomography, multislice computed tomography, coronary artery disease
Selective coronary angiography remains the gold standard for the diagnosis of coronary arterial stenosis severity. However, approximately 40–50% of all coronary angiograms are done to exclude significant stenoses and are not followed by an intervention. As this invasive procedure is costly and is not without risk, an alternative non-invasive technique would be of great benefit.1–4 To date, promising attempts have been made to visualise the coronary arteries non-invasively with electron beam computed tomography (EBCT) and magnetic resonance imaging.5–12 Because of its low temporal resolution, conventional mechanical computed tomography was not able to generate motion-free images of the heart. The recent development of helical multislice computed tomography (MSCT) has now made such an achievement possible, owing to a major reduction in acquisition time.13–15
In contrast to the fast expanding MSCT technique, EBCT is only available in specialised centres. Thus our aim in this study was to compare MSCT coronary angiography (MSCTA) with EBCT coronary angiography (EBCTA) for diagnostic accuracy and image quality.
METHODS
Patients
We investigated 101 consecutive patients (21 female, 80 male) with EBCTA within six days of selective coronary angiography between April 1998 and January 2000; an additional series of 91 consecutive patients was studied with MSCTA (19 female, 72 male) between July 1999 and May 2001. All patients were referred for evaluation of typical or atypical angina pectoris or abnormal results in non-invasive physiological tests for ischaemia. Exclusion criteria for both investigations were atrial fibrillation, renal insufficiency with a plasma creatinine of more than 133 μmol/l, unstable clinical condition, and known or suspected iodine allergy. The protocols were approved by an institutional review board and informed consent was obtained from all the patients. The characteristics of the patients and their lesions are given in table 1.
Table 1.
Characteristics of the patients and their coronary lesions
| MSCTA | EBCTA | |
| Angina pectoris | 56 | 70 |
| Evaluation after MI | 18 | 13 |
| Previous angioplasty | 11 | 8 |
| Valvar disease | 2 | 7 |
| Cardiomyopathy | 2 | 3 |
| 1 Vessel disease | 32 | 25 |
| 2 Vessel disease | 24 | 15 |
| 3 Vessel disease | 6 | 18 |
| No stenosis | 30 | 53 |
| Age (years) | 61.7 (11.0) | 59.2 (9.1) |
| Body mass index (kg/m2) | 26.4 (3.0) | 27.0 (3.0) |
| Heart rate (beats/min) | 64 (7) | 68 (11) |
| Volumetric calcium score | 269 (388) | 288 (417) |
| Stenosis in LM | 5 | 5 |
| Stenosis in LAD | 44 | 48 |
| Stenosis in LCx | 13 | 15 |
| Stenosis in RCA | 21 | 20 |
| Mean coronary diameter (mm) | 3.3 (1.2) | 3.1 (1.4) |
Values are n or mean (SD).
Differences between the groups are not significant.
EBCTA, electron beam computed tomographic coronary angiography; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LM, left main coronary artery; MI, myocardial infarction; MSCTA, multislice computed tomographic coronary angiography; RCA, right coronary artery.
Selective coronary angiography
Coronary angiography was done using the Judkins technique. On average six views were obtained, including two pairs of orthogonal views. These were analysed by two independent experienced cardiologists unaware of the results of the computed tomography. For each stenosis the maximum per cent lumen narrowing was determined visually.
Electron beam computed tomographic angiography
For EBCTA we used an Imatron C-150 XP scanner (Imatron Inc, San Francisco, California, USA). Before EBCTA a calcium scoring scan was done and the volumetric calcium score determined.16 A previously described standardised protocol which is widely used for EBCTA was employed in this study.5–10 After a delay equal to the contrast media transit time, 50 cardiac images were acquired. A collimation of 3 mm and table increment of 2 mm generated overlapping 3 mm slices. We optimised spatial resolution by selecting the smallest possible field of view and tried to reduce motion artefacts, especially of the right coronary artery, by determining the optimal ECG trigger interval for each patient. For that purpose we chose the scan position with the most pronounced motion artefacts in the calcium screening scan, which was done at 80% of the RR interval. At that same position we did three further single slice scans at 50%, 60%, and 70% of the RR interval and chose the trigger point providing the least motion artefact in each patient for subsequent scanning.
Multislice computed tomographic angiography
The major difference between the new generation MSCT and conventional mechanical spiral computed tomography scanners is the presence of four detector rows, which allows four slices to be generated simultaneously. In addition, the tube rotation time can be reduced to 500 ms. Thus by using retrospective ECG triggering and a partial 180° scan technique, effective temporal resolution can be reduced to 250 ms per slice.
For MSCTA we used a Siemens Somatom Plus 4 volume zoom scanner (Siemens Medical Systems, Forchheim, Germany) supplied with special cardiac reconstruction software. We applied a similar algorithm and the same software as recently described by Niemann and colleagues.17
The transition time of contrast media was determined by injecting a test bolus (20 ml, flow rate 3 ml/s) and repeated scanning (one scan every 1.5 s) at the level of the aortic root. Following the intravenous injection of 140 ml of contrast media (flow rate 3 ml/s) and after the predetermined transit delay, scanning was started at a level just below the carina. The entire volume of the heart was covered in a spiral technique with simultaneous digital registration of the ECG signal during one breath hold (mean (SD), 32 (6) s). The collimation of 4 * 1 mm and a table feed of 3 mm/s permitted a later reconstruction of overlapping slices 1.25 mm thick. The tube current was 300 mA at 120 kV, providing an optimal ratio between image noise and radiation dose.
For retrospective ECG gating the raw data were transferred to an external workstation to be reconstructed in (mean (SD)) 215 (12) overlapping slices (slice thickness 1.25 mm, overlap 0.5 mm) at any time during the RR interval. We reconstructed all images between 300–470 ms before the R wave in diastole to prevent severe motion artefacts, as previously described.18 In initial investigations we found severe motion artefacts during rapid heart rates.19 We therefore treated all patients who had heart rates of more than 70 beats/min with 50 mg oral metoprolol (n = 18) or with 40–100 mg intravenous esmolol (n = 8). Technical details of EBCTA and MSCTA are given in table 2.
Table 2.
Technical details of electron beam computed tomography (EBCT) and multislice computed tomography (MSCT)
| Parameter | EBCT | MSCT |
| Spacial resolution | 6 line pairs/cm | 9 line pairs/cm |
| Acquisition time | 100 ms | 250 ms |
| Radiation dose | 2.7 mSv | 8.2 mSv |
| ECG trigger | Prospective | Retrospective |
| Slice thickness | 3 mm | 1.25 mm |
| Overlap | 1 mm | 0.5 mm |
Correlation of selective coronary angiography with the results of EBCTA and MSCTA
Analysis of the EBCTA and MSCTA datasets was done independently by a cardiologist (AL) and a radiologist (CB) using the source images, with maximum intensity projection and volume rendering reconstructions provided by an external workstation (Insight, Neo Imagery, California, USA). Before analysis, all segments were graded as to whether or not they were assessable on the basis of the contrast to noise ratio (> 3.0) and the presence or absence of motion artefacts. In case of non-consensus a joint reading was undertaken.
EBCT and MSCT scans were not done during the same time period and were partly separated by almost two years. To prevent the influence of a learning effect on the later results, we analysed all the MSCT and EBCT data at one time, after all the studies had been done. For correlation with selective coronary angiography, the coronary tree was divided into segments according to the American Heart Association guidelines.20 Only segments 1–3 of the right coronary artery, segments 5, 6, 7, 8 of the left coronary artery, and segment 11 (circumflex artery), which was divided into a proximal and a distal part, were analysed for stenoses of more than 50% (the nine segment model). This method has been used previously in studies of EBCTA and MSCTA.5–10,15,17 Angiograms were analysed by two experienced cardiologists, who were blinded to the findings in MSCTA or EBCTA, using the Accom PC software package (Siemens). All stenoses found on EBCTA/MSCTA and coronary angiography were assigned to the same standardised diagram and compared on a site by site basis. Using coronary angiography as the gold standard, sensitivity, specificity, and accuracy were calculated. Coronary segments distal to a vessel occlusion and segments with a coronary stent were not considered for analysis.
Radiation dose
The radiation dose for EBCTA and MSCTA was calculated using commercially available Win Dose software. For EBCTA an average dose of approximately 2.4 mSv and for MSCTA approximately 8.2 mSv was calculated.
Determination of the contrast to noise ratio
To obtain an objective index of image quality we determined the contrast to noise ratio for both EBCTA and MSCTA for all the predetermined coronary segments. To do this, we measured the mean density within the lumen of all segments and in the connective tissue immediately next to the coronary vessel and divided the Houndsfield unit difference by the image noise, which is determined as the standard deviation of the density value measured in a region of interest in the aortic root.
Statistics
Statistical calculations were done using the SPPS software package (version 10.0) installed in a desktop computer. Numerical variables were compared using the Wilcoxon test and the Student t test. Proportions were compared by determining the 95% confidence interval. If the confidence intervals in the two groups did not overlap in a particular comparison, the difference was considered to be significant. A probability value of p < 0.05 was regarded as significant. These calculations were done by using Windows Excel 2000 (version 9.0).
RESULTS
Age, calcium score, and body mass index were similar in patients investigated by EBCTA and MSCTA (table 1). The heart rate in the EBCTA group was slightly higher than in the MSCTA group, although the difference was not significant. Mean coronary vessel diameter, which was measured in all vessels in all patients on the tomographic images, did not differ between the two groups. The distribution of stenoses in the different assessable segments between the two groups was also similar (table 1).
Electron beam computed tomographic angiography
In four patients, either the left or the right coronary artery was not investigated by coronary angiography (18 segments); furthermore 15 segments were excluded because of a proximal occlusion or a coronary stent. Thus in the 101 patients investigated with EBCTA, 876 of the 909 predetermined coronary segments (in the nine segment model) were included for analysis. Of these remaining 876 segments, 662 (76%) were assessable by EBCTA with sufficient diagnostic image quality.
The main reasons why poor image quality made assessment impossible were a low contrast to noise ratio in 102 segments and motion artefacts caused by respiration (n = 27) and cardiac movement (n = 79). Six segments could not be assessed because the scan started too low (table 3). Of the 98 angiographically documented significant stenoses (> 50%), 64 could be detected with EBCTA. Fifteen stenoses were located in segments not assessable by EBCTA, and 19 stenoses that were located in assessable segments were overlooked. Overall, stenoses were correctly ruled out in 537 of 778 segments. In 40 segments EBCTA revealed false positive results, and 201 segments without an obstruction were not assessable. The overall sensitivity and specificity respectively of EBCTA for the detection and the exclusion of coronary stenoses present only in segments providing adequate image quality is given in table 4. The best accuracy was achieved in the left main coronary artery, followed by the left anterior descending, right, and circumflex coronary arteries. The contrast to noise ratio in the different coronary segments is given in table 5. As expected it decreased significantly from proximal to distal vessels.
Table 3.
Number of assessable segments with electron beam computed tomographic coronary angiography (EBCTA) and multislice computed tomographic coronary angiography (MSCTA)
| EBCTA | MSCTA | ||||
| Segment | Number assessable | % (95% CI) | Number assessable | % (95% CI) | p Value |
| 1 | 75/99 | 76% (66% to 84%) | 75/88 | 85% (76% to 92%) | NS |
| 2 | 72/99 | 73% (62% to 81%) | 56/88 | 64% (53% to 74%) | NS |
| 3 | 53/92 | 58% (46% to 67%) | 62/85 | 73% (62% to 82%) | NS |
| 5 | 87/101 | 86% (78% to 92%) | 84/91 | 92% (84% to 96%) | NS |
| 6 | 81/98 | 83% (74% to 90%) | 78/87 | 90% (81% to 95%) | NS |
| 7 | 81/100 | 81% (72% to 88%) | 75/88 | 85% (76% to 92%) | NS |
| 8 | 73/100 | 73% (63% to 81%) | 78/90 | 87% (79% to 92%) | NS |
| 11 | 79/96 | 82% (73% to 89%) | 78/89 | 88% (78% to 93%) | NS |
| 11 distal | 61/91 | 67% (56% to 77%) | 67/89 | 75% (65% to 83%) | NS |
| Total | 662/876 | 76% (72% to 78%) | 653/795 | 82% (79% to 84%) | <0.05 |
CI, confidence interval.
Table 4.
Sensitivity and specificity for the detection of coronary stenoses exceeding 50% with electron beam computed tomographic coronary angiography (EBCTA) and multislice computed tomographic coronary angiography (MSCTA)
| Sensitivity | Specificity | |||||||
| EBCTA | MSCTA | EBCTA | MSCTA | |||||
| Segment | n* | % (95% CI) | n* | % (95% CI) | n† | % (95% CI) | n† | % (95% CI) |
| 1 | 7/8 | 88% (47% to 100%) | 11/13 | 85% (55% to 98%) | 59/67 | 88% (80% to 97%) | 58/62 | 94% (85% to 98%) |
| 2 | 5/10 | 50% (19% to 81%) | 4/6 | 67% (22% to 96%) | 58/62 | 94% (84% to 98%) | 47/50 | 94% (83% to 99%) |
| 3 | 2/3 | 67% (9% to 99%) | 1/1 | 100% (45% to 100%) | 49/50 | 98% (89% to 100%) | 59/61 | 97% (89% to 100%) |
| 5 | 4/5 | 80% (28% to 99%) | 4/5 | 80% (28% to 99%) | 80/82 | 98% (89% to 100%) | 78/79 | 99% (93% to 99%) |
| 6 | 16/17 | 94% (71% to 99%) | 20/23 | 87% (66% to 97%) | 59/64 | 92% (91% to 100%) | 51/55 | 93% (82% to 98%) |
| 7 | 18/19 | 95% (74% to 100%) | 18/20 | 90% (68% to 99%) | 54/62 | 87% (76% to 94%) | 52/55 | 95% (85% to 99%) |
| 8 | 4/8 | 50% (16% to 84%) | 5/5 | 100% (47% to 100%) | 59/65 | 91% (81% to 97%) | 70/73 | 96% (88% to 99%) |
| 11 | 8/12 | 67% (34% to 90%) | 8/12 | 67% (34% to 90%) | 60/67 | 90% (80% to 96%) | 64/66 | 97% (90% to 100%) |
| 11 distal | 0/1 | – | 1/3 | 33% | 59/60 | 98% (91% to 100%) | 60/64 | 94% (84% to 98%) |
| Total | 64/83 | 77% (67% to 86%) | 72/88 | 82% (72% to 89%) | 537/579 | 93% (90% to 95%) | 539/565 | 96% (94% to 97%) |
*Sensitivity, number detected/number present on coronary angiography; †specificity, number excluded/number not present on coronary angiography.
Differences between EBCTA and MSCTA are not significant in any segment.
CI, confidence interval.
Table 5.
Contrast to noise ratio of electron beam computed tomographic coronary angiography (EBCTA) and multislice computed tomographic coronary angiography (MSCTA)
| Segment | EBCTA | MSCTA |
| 1 | 7.6 (2.3) | 11.6 (2.1) |
| 2 | 6.6 (3.0) | 8.7 (2.3) |
| 3 | 4.9 (3.3) | 8.2 (2.9) |
| 5 | 8.6 (2.2) | 12.6 (3.8) |
| 6 | 7.7 (2.3) | 10.8 (3.9) |
| 7 | 6.6 (2.6) | 9.7 (2.6) |
| 8 | 5.0 (2.8) | 6.7 (3.2) |
| 11 | 6.4 (2.2) | 9.7 (4.2) |
| 11 distal | 5.0 (2.0) | 6.2 (3.8) |
| Mean CNR | 6.5 (2.3) | 9.4 (4.9) |
Values are mean (SD).
The differences between the two groups are significant in each segment (p value at least <0.05).
CNR, contrast to noise ratio.
Multislice computed tomographic angiography
With MSCTA, 26 of the 91 patients included were treated with a β blocker because they had a heart rate of more than 70 beats/min. The reduction in heart rate in these patients (mean (SD)) was 9 (6) beats/min.
Of 819 segments in 91 patients, 795 were included for analysis. Overall, 24 segments were not included for analysis because they could not be investigated by coronary angiography (n = 3) or because there was a proximal vessel occlusion or a coronary stent (n = 21). Thus 795 of 819 segments in 91 patients were considered for analyses. Of these 795 segments, 653 could be assessed by MSCTA with appropriate image quality (table 3).
Evaluation was made impossible in 96 segments by cardiac motion artefacts, in 17 by respiration artefacts, and in 25 by a poor contrast to noise ratio. Four segments could not be visualised because the scan started too low (table 3). Overall, 72 of 107 coronary stenoses of more than 50% could be detected with MSCTA. Nineteen stenoses were not detected because they were localised in segments with poor image quality, and 16 stenoses in assessable segments were overlooked. In 539 of 698 segments, stenoses of more than 50% were correctly ruled out. Thus 134 segments could not be assessed because of poor image quality and in 25 assessable segments there were false positive results. The values for diagnostic sensitivity and specificity in the segments with adequate image quality are given in table 4. The contrast to noise ratios for the various coronary segments are given in table 5.
Comparison of MSCTA and EBCTA
MSCTA allowed the visualisation of significantly more predetermined segments than EBCTA (82% v 76%; p < 0.05). With respect to sensitivity and specificity, the difference between MSCTA and EBCTA was non-significant. The contrast to noise ratio of MSCTA was significantly higher in all segments investigated (p < 0.05).
Examples of images obtained using EBCTA and MSCTA are shown in figs 1, 2, and 3.
Figure 1.
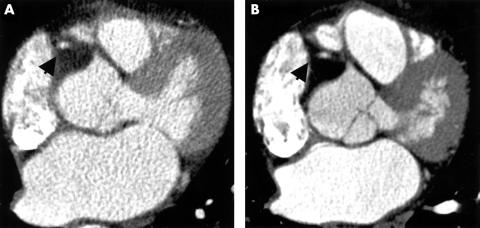
Electron beam computed tomographic coronary angiography (EBCTA; panel A) and multislice computed tomographic coronary angiography (MSCTA; panel B) in the same 62 year old patient. On this axial image at the level of the proximal right coronary artery (RCA) the differences in contrast to noise ratio (CNR) and spatial resolution between the two methods are visualised. The arrow indicates the right coronary artery.
Figure 2.
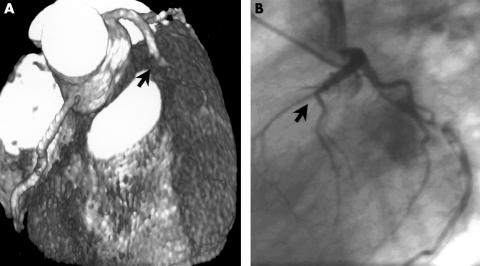
Proximal occlusion of the left anterior descending coronary artery (LAD) in a 52 year old patient with atypical angina pectoris (arrow). (A) Electron beam computed tomographic coronary angiography (EBCTA), reconstructed with volume rendering. (B) Conventional coronary angiography.
Figure 3.
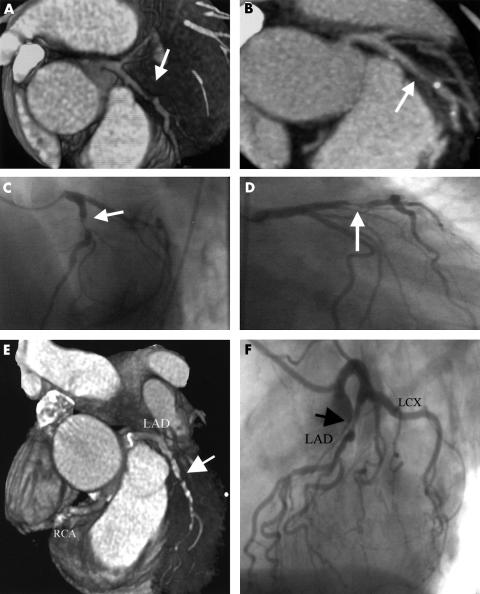
Panels A to D, high grade stenosis of the left anterior descending coronary artery (LAD) (arrow) in a 62 year old woman with angina pectoris: (A) multislice computed tomographic coronary angiography (MSCTA), volume rendering reconstruction; (B) MSCTA, maximum intensity projection; (C) conventional coronary angiography, left anterior oblique projection; (D) conventional coronary angiography, right anterior oblique projection. Panels E and F, high grade stenosis of the LAD in a 57 year old woman: (E) MSCTA, volume rendering; (F) conventional coronary angiography.
DISCUSSION
This is the first study comparing diagnostic accuracy and image quality between MSCTA and EBCTA in the coronary vessels in different but comparable patient cohorts. Our results show that in this setting both MSCTA and EBCTA achieve similarly accurate visualisation of the coronary anatomy and a similar rate of detection of stenoses. With MSCTA, more coronary segments can be visualised with good image quality than with EBCTA, because the contrast to noise ratio and spatial resolution are better with MSCTA. However, those advantages are offset by a greater radiation exposure. Both methods have clinical limitations in that a moderate number of coronary segments cannot be assessed at all.
Comparison of EBCTA and MSCTA
EBCTA has been evaluated by various investigators.5–10 In most studies, high sensitivities (range 93–78%) and high specificities (range 98–88%) for the detection of significant coronary stenoses have been reported. These results are consistent with those of our study. MSCTA allowed the detection of coronary stenoses with a similar sensitivity and specificity to EBCTA. However, with both methods these sensitivities and specificities could only be achieved after a substantial number of segments had been excluded because of poor image quality. More segments were assessable with MSCTA than with EBCTA (82% v 76%; p < 0.05). As in previous published studies, 20–28% of coronary segments had to be excluded from evaluation in the EBCTA data. For MSCTA, however, only limited previous data are available. Niemannn and colleagues found that 76% of coronary segments could be evaluated,17 and Achenbach and associates, 60%.21 The higher percentage of assessable segments in our study may be explained by the fact that in both those former studies heavily calcified segments were excluded and β blockers were not used to reduce the heart rate. Niemann and colleagues applied a similar scan algorithm and protocol to the one we used in the present study. In contrast, Achenbach and associates used a protocol with a higher tube energy (140 kV v 120 kV) but a lower current (190 mA v 300 mA) and a different scan algorithm. These differences may have resulted in a lower contrast to noise ratio and decreased the image quality. In a previous report from our group it was shown that in a carefully preselected and prepared patient cohort, consistent image quality (94% of coronary segments assessable) and high diagnostic accuracy (92%) can be achieved with MSCTA.15 However, as indicated by the results of the present study—which was done without preselection in consecutive patients—these findings cannot generally be transferred to real world conditions.
Despite better spatial in-plane and z axis resolution and a higher contrast to noise ratio, the higher diagnostic accuracy of MSCTA compared with EBCTA is only marginal and not significant. This probably reflects the fact that the majority of coronary stenoses are located in proximal coronary segments. In these segments the image quality of EBCTA is also high, allowing an accurate detection of significant stenoses.
The major limitation of MSCTA remains the relatively slow acquisition time, leading to extensive motion artefacts in patients with heart rates exceeding 70 beats/min and making pretreatment with negative chronotropic agents necessary. However, with EBCTA we also observed a rather large number of motion artefacts caused by cardiac movement. Variations in heart rate occurring within one breath hold, caused by extrasystoles and other arrhythmias, may result in irreversible motion artefacts in a prospective ECG gated scan such as is used with EBCTA.22,23 The reason why our patients investigated with EBCT were not also treated with a β blocker is that with EBCT and prospective triggering only one slice per heart beat can be acquired, and for scanning the whole heart at least 40 slices are necessary. Therefore slow heart rates increase the total EBCT scan time and provoke breathing artefacts.
Retrospective ECG gating permits the reconstruction of images at identical time points within the RR interval, regardless of changes in the sinus rate or the presence of arrhythmias. Furthermore, different trigger points for each coronary artery can be selected. This may be important as coronary motion is most variable in the major coronary vessels.18 Retrospective ECG gating can therefore partly compensate for the longer acquisition time and may become the favoured trigger technique. However, it seems to be necessary to develop scanners with acquisition times below 100 ms to fully compensate for the rapid motion of the coronary arteries and to achieve diagnostic image quality in a larger proportion of patients.
Another significant limitation relevant to MSCTA is the 3.5-fold greater radiation dose compared with EBCT. The reason for this is that radiation is applied continuously during the entire heart cycle, though only data acquired during diastole are used for image reconstruction. This limitation will be improved by developments involving synchronisation of the tube current to the ECG so as to limit radiation to diastole. However, at present it is probable that in certain instances—for example, obesity—the radiation dose may exceed the 8.2 mSv that was calculated in our study.
With both methods we found it possible to visualise large portions of the left coronary artery with high sensitivity and high specificity. In contrast, sensitivity for the circumflex artery and specificity for the right coronary artery were low with both methods. This is likely to reflect differences in the anatomical course of these two vessels. The mid-right coronary artery, which has the greatest motion during the cardiac cycle, runs perpendicular to the axial slice and is especially sensitive to motion artefacts caused by arrhythmias or inaccurate triggering. These artefacts may resemble stenoses, and in our study they led to several false positive results. The poorest sensitivity with both modes was observed for the distal part of the circumflex artery. Because its course runs very close to the great coronary vein and its diameter is often small, stenoses here can easily be overlooked.
Coronary calcification is a known pitfall for computed tomographic angiography. Because of the high density of calcium—which is either greater than or in the range of the contrast enhanced coronary lumen—stenoses sometimes cannot be accurately visualised. We have already shown in a previous EBCT study that the diagnostic accuracy of EBCTA is inferior in the presence of high coronary calcium scores.24 In our present study, 10 of 61 false positive and false negative results in the EBCTA group (16%) were caused by vessel calcification, and 7 of 42 (17%) in the MSCTA group. However, for both methods this is only a minor limitation as high calcium scores are associated with a high prevalence of coronary stenoses. For future clinical purposes, computed tomographic angiography should only be done after excluding extensive calcification.
Limitations
Both EBCTA and MSCTA require the administration of contrast media and x ray exposure. Institutional ethical guidelines prevented us from doing both investigations in the same patient. Our study therefore represents an indirect comparison. However, both investigations were done under similar clinical conditions in patients with similar physical and clinical characteristics (table 1). Diagnostic accuracy and assessability of both diagnostic tests are influenced by specific lesion characteristics, lesion location, and vessel anatomy. In different patient cohorts these influences cannot totally be eliminated. However, the distribution of stenoses in the different coronary segments and the coronary calcium score indicating lesion morphology were similar in our two groups (table 1).
Coronary stenoses on x ray angiography were estimated visually, which is inferior to quantitative coronary angiography. However, evaluation of the angiograms was always done by the same two experienced interventional cardiologists.
Abbreviations
EBCT, electron beam computed tomography
EBCTA, electron beam computed tomographic coronary angiography
MSCT, multislice computed tomography
MSCTA; multislice computed tomographic coronary angiography
REFERENCES
- 1.Anon. Report on performance figures in the cardiac catheterisation laboratories in Germany. Z Kardiol 2000;89:976–8. [Google Scholar]
- 2.American Heart Association. Heart and stroke facts: 1995 statistical supplement. Dallas, Texas: American Heart Association, 1995.
- 3.Adams DF, Fraser DB, Abrams HL. The complications of coronary arteriography. Circulation 1973;48:609–18. [DOI] [PubMed] [Google Scholar]
- 4.Levin DC. Invasive evaluation (coronary arteriography) of the coronary artery disease patient: clinical, economic and social issues. Circulation 1982;66(suppl III):71–9. [PubMed] [Google Scholar]
- 5.Budoff MJ, Oudiz RJ, Zalace CP, et al. Intravenous three-dimensional coronary angiography using contrast enhanced electron beam computed tomography. Am J Cardiol 1999;83:840–5. [DOI] [PubMed] [Google Scholar]
- 6.Moshage WE, Achenbach S, Seese B, et al. Coronary artery stenoses: three-dimensional imaging with electrocardiographically triggered, contrast agent-enhanced, electron-beam CT. Radiology 1995;196:707–14. [DOI] [PubMed] [Google Scholar]
- 7.Reddy GP, Chernoff DM, Adams JR, et al. Coronary artery stenoses: assessment with contrast-enhanced electron-beam CT and axial reconstructions. Radiology 1998;208:167–72. [DOI] [PubMed] [Google Scholar]
- 8.Schmermund A, Rensing BJ, Sheedy PF, et al. Intravenous electron-beam CT coronary angiography for segmental analysis of coronary artery stenoses. J Am Coll Cardiol 1998;31:1547–54. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach S, Moshage W, Ropers D, et al. Value of electron beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N Engl J Med 1998;339:1964–71. [DOI] [PubMed] [Google Scholar]
- 10.Rensing BJ, Bongaerts A, van Geuns RJ, et al. Intravenous coronary angiography by electron beam computed tomography: a clinical evaluation. Circulation 1998;98:2509–12. [DOI] [PubMed] [Google Scholar]
- 11.Sandstede J, Pabst T, Beer M. Three dimensional MR coronary angiography using navigator technique compared with conventional coronary angiography. Am J Radiol 1999;172:135–9. [DOI] [PubMed] [Google Scholar]
- 12.Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med 2001;345:1863–9. [DOI] [PubMed] [Google Scholar]
- 13.Ohnesorge B, Flohr T, Becker C, et al. Cardiac imaging using retrospective ECG-gated multi detector CT. Radiologe 2000;2:111–17. [DOI] [PubMed] [Google Scholar]
- 14.Becker CR, Knez A, Leber A, et al. First experience using multidetector CT for evaluation of coronary atherosclerosis. Radiologe 2000;2:118–22. [Google Scholar]
- 15.Knez A, Becker CR, Leber A, et al. Usefulness of multislice spiral computed tomography angiography for determination of coronary artery stenoses. Am J Cardiol 2001;88:1191–4. [DOI] [PubMed] [Google Scholar]
- 16.Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron beam CT volumetric method. Radiology 1998;208:807–14. [DOI] [PubMed] [Google Scholar]
- 17.Niemann K, Oudkerk M, Rensing BJ, et al. Coronary angiography with multi-slice computed tomography. Lancet 2001;357:599–603. [DOI] [PubMed] [Google Scholar]
- 18.Achenbach S, Ulzheimer S, Baum U, et al. Noninvasive coronary angiography by retrospectively gated multislice spiral CT. Circulation 2000;102:2823–8. [DOI] [PubMed] [Google Scholar]
- 19.Hong C, Becker CR, Bruening RD, et al. Retrospective ECG gating technique for reduction of cardiac motion artifacts in multislice CT coronary angiography. Radiology 2001;217:374. [Google Scholar]
- 20.American Heart Association. Committee report. A reporting system on patients evaluated for coronary artery disease. Circulation 1975;51:7–34. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach S, Giesler T, Ropers D, et al. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography. Circulation 2001;103:2535–8. [DOI] [PubMed] [Google Scholar]
- 22.Mao S, Oudiz R, Bakhsheshi H, et al. Variation of the heart rate and electrocardiographic trigger interval during ultrafast computed tomography. Am J Card Imaging 1996;10:239–43. [PubMed] [Google Scholar]
- 23.Achenbach S, Ropers D, Holle J, et al. In-plane coronary arterial motion velocity: measurement with electron-beam CT. Radiology 2000;216:457–63. [DOI] [PubMed] [Google Scholar]
- 24.Leber AW, Knez A, Mukherjee R, et al. Usefulness of calcium scoring using electron beam computed tomography and noninvasive coronary angiography in patients with suspected coronary artery disease. Am J Cardiol 2001;88:219–23. [DOI] [PubMed] [Google Scholar]