New York Heart Association (NYHA) classification is popularly used to evaluate severity of stable chronic heart failure. Since this evaluation depends on subjective symptoms such as dyspnoea or fatigue with exertion, it is not expected to provide a precise reflection of physiologic abnormality. Useful objective indices to evaluate severity of heart failure are peak oxygen uptake (V̇o2) and minute ventilation–carbon dioxide output (V̇E–V̇co2) slope, which are derived from cardiopulmonary exercise testing,1 but the required methodology is complex and may not be widely available. Previous studies have shown that plasma brain natriuretic peptide (BNP), a cardiac hormone secreted mainly by cardiac ventricles, increases in chronic heart failure and correlates with haemodynamic abnormalities such as left ventricular dysfunction.2 Although measurement of plasma BNP has been thought of as a simple way to diagnose heart failure,2 it has not been revealed how well plasma BNP concentration predicts severity of heart failure. If exercise tolerance could be shown to correlate with plasma BNP concentration in heart failure, it would enhance this measurement’s value in evaluating heart failure severity. In this study we investigated the relation between plasma BNP concentration and exercise tolerance in patients with chronic stable heart failure.
METHODS
Patients with stable chronic heart failure (neither diuretics nor inotropic drug were changed within two weeks before the study and more than four weeks had elapsed after an acute heart failure episode requiring hospital admission) with left ventricular ejection fraction (LVEF) ≤ 40% were enrolled. Patients with pacemaker, severe lung disease, acute metabolic disorder, serum creatinine 265 μmol/l, acute myocardial infarction within one month, post-cardiovascular surgical operation within one month, or moderate to severe aortic stenosis were excluded. In total, 38 chronic heart failure patients (27 men and 11 women; mean (SD) age 64 (9); 18 with dilated cardiomyopathy, 16 with old myocardial infarction, and four with other heart diseases) were enrolled. LVEF was 28.1 (7.5)%.
Blood samples were obtained before exercise testing and were centrifuged, plasma was separated and stored at −20° until assayed. Plasma BNP concentrations were assessed with a highly sensitive radioimmunoassay kit (Shionoria BNP, Japan). Patients performed a symptom limited, incremental exercise test using an upright, calibrated, electromagnetically braked cycle ergometer (CPE2000, Medical Graphics Co, Minnesota, USA) with 12 lead ECG monitoring. This test began with three minutes of rest and three minutes of warm up of 0 W cycling, followed by a continuous increase in work rate of 10 W per minute. During exercise, patients wore a facemask and the respired gas was analysed; V̇o2, V̇co2, and V̇E were calculated for each breath utilising a metabolic cart (AE280S, Minato Co, Tokyo, Japan).
Comparisons of plasma BNP concentration between cardiac function groups were analysed with the Kruskal-Wallis test and the Mann-Whitney U test. Single and multiple regression analyses were used to assess the association of plasma BNP concentration and cardiopulmonary indices. A probability value of p < 0.05 was regarded as significant.
RESULTS
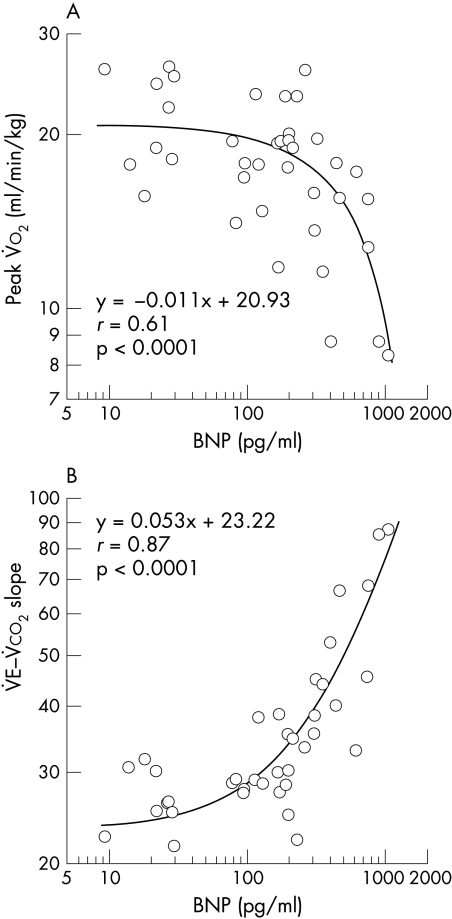
Mean (SD) plasma BNP concentration was 252.8 (260.3) pg/ml (median 181.6 pg/ml). Plasma BNP concentration significantly correlated with LVEF (r = −0.36, p = 0.04). Peak V̇o2 (18.1 (4.8) ml/min/kg) and V̇E–V̇co2 slope (36.7 (15.9)) were determined in all patients. We could not determine anaerobic threshold in four patients with very low exercise tolerance (three patients) or paroxysmal atrial tachycardia during exercise (one patient). According to the heart function classification by peak V̇o2 of Weber and colleagues,1 subjects can be assigned to four groups (class A, B, C, and D). Plasma BNP concentrations were as follows: class A (peak V̇o2 > 20 ml/min/kg, n = 10)—111.0 (100.3) pg/ml; class B (16 ml/min/kg < peak V̇o2 = 20 ml/min/kg, n = 15)—185.8 (166.6) pg/ml; class C (9 ml/min/kg < peak V̇o2 = 16 ml/min/kg, n=10)— 334.7 (259.4) pg/ml; class D (peak V̇o2 = 9 ml/min/kg, n = 3)—787.6 (338.0) pg/ml. Mean BNP concentration significantly increased with decreasing exercise tolerance (Kruskal-Wallis test, p = 0.013). Plasma BNP concentration significantly correlated with peak V̇o2 and V̇E–V̇co2 slope (fig 1). Stepwise regression analysis of plasma BNP concentration and exercise testing indices except anaerobic threshold showed that the V̇E–V̇co2 slope alone explained 74% of the variance of plasma BNP concentration.
Figure 1.
Relation between plasma brain natriuretic peptide (BNP) and (A) peak V̇o2 or (B) V̇E–V̇co2 slope.
DISCUSSION
Plasma BNP is useful in diagnosing heart failure, with high plasma BNP concentrations predicting a worse prognosis.3 Earlier studies did not give specific information regarding what concentrations of plasma BNP predict a worse prognosis because most studies divided subjects into high or low BNP groups by median BNP value. These studies can only indicate whether patients with high plasma BNP concentrations have clinical or subclinical heart failure, but cannot show how severe heart failure was. NYHA classification of heart failure and plasma BNP concentration are correlated,4 but a useful prediction of exercise tolerance from plasma BNP remains to be seen. We sought a clear relation between plasma BNP concentration and objective severity of heart failure. Peak V̇o2 from cardiopulmonary exercise testing is an objective estimator of heart failure severity.1 Recently, it was reported that high values of V̇E–V̇co2 slope and exercise V̇E/V̇co2 have a similar ability to evaluate heart failure severity.5 Plasma BNP concentration strongly correlates with exercise tolerance (such as peak V̇o2 and V̇E–V̇co2 slope) among heart failure patients, thus enhancing its use in evaluating patients with heart failure.
In this study, we found strong correlations between V̇E–V̇co2 slope and plasma BNP concentration. The data in fig 1B shows that a V̇E–V̇co2 slope < 34 (upper 95% confidence limit of normal subjects4) corresponds to a plasma BNP concentration < 200 pg/ml (specificity 0.87, sensitivity 0.80). Figure 1 clearly shows that a plasma BNP concentration around 200 pg/ml is the break point for development of exercise intolerance. Patients with V̇E–V̇co2 slope > 61 (upper 95% confidence limit of chronic heart failure4) generally had a BNP concentration > 700 pg/ml (specificity 0.97, sensitivity 0.75); two of four patients with plasma BNP concentrations > 700 pg/ml died within a year after they enrolled in this study. Plasma BNP < 200 pg/ml would predict nearly normal exercise tolerance in heart failure patients, and plasma BNP > 700 pg/ml would be the critical value for predicting high mortality and exercise intolerance for heart failure patients. This correlation of plasma BNP with exercise tolerance is very useful as it allows objective determination of a patient’s heart failure severity without having to perform exercise testing.
Abbreviations
BNP, brain natriuretic peptide
LVEF, left ventricular ejection fraction
NYHA, New York Heart Association
V̇co2, carbon dioxide output
V̇E, minute ventilation
V̇o2, oxygen uptake
REFERENCES
- 1.Weber KT, Wilson JR, Janicki JS, et al. Exercise testing in the evaluation of the patient with chronic cardiac failure. Am Rev Respir Dis 1984;129:S60–2. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimura M, Yasue H, Morita E, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 1991;84:1581–8. [DOI] [PubMed] [Google Scholar]
- 3.Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997;96:509–516. [DOI] [PubMed] [Google Scholar]
- 4.Chuna TP, Ponikowski P, Harrington D, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585–90. [DOI] [PubMed] [Google Scholar]
- 5.Kleber FX, Vietzke G, Wernecke KD, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation 2000;101:2803–9. [DOI] [PubMed] [Google Scholar]