It has recently been recognised that adipose tissue, which accounts for more than 10% of body weight, is not only a reservoir for energy storage, but is also an endocrine tissue.1 Adiponectin, which is structurally related to collagen, is a novel adipose specific gene product that circulates at high concentrations.2 Adiponectin has an anti-inflammatory effect on endothelial cells, inhibits the proliferation of vascular smooth muscle cells, and suppresses the conversion of macrophages to foam cells.1 Plasma adiponectin concentrations are significantly lower in obese subjects than in non-obese subjects, significantly lower in patients with coronary artery disease (CAD) than in control subjects, and associated with insulin resistance in animal models.2–4
Cigarette smoking is one of the major coronary risk factors for CAD. Indeed, major epidemiological prospective studies have unequivocally demonstrated the relation between cigarette smoking and CAD mortality, and that smoking cessation reduces mortality from CAD.5 There are several pathogenic mechanisms proposed to explain the harmful effects of smoking on CAD, such as stimulation of the sympathetic nerve system, vasoconstriction, endothelial injury, and dyslipidaemia.5 However, the atherogenic mechanisms of smoking have not been fully determined. Here, we present our findings of an association between adiponectin and smoking status in patients with CAD.
METHODS
We examined 127 consecutive patients (111 men, mean age 61.8 years) with documented CAD defined as more than 75% stenosis by coronary angiography. All patients gave their informed consent. All blood samples were drawn just before coronary angiography. Plasma concentrations of adiponectin were measured by enzyme linked immunosorbent assay.2 Former smoker was defined as a person who had abstained from smoking for more than one year. To assess insulin sensitivity, we used the parameter of homeostasis model assessment insulin resistance (HOMA-IR: fasting plasma glucose (mM) × fasting insulin (μU/ml)). Statistical differences between the groups were analysed by one way analysis of variance (ANOVA) and χ2 test. Correlation between the two parameters was determined by simple linear regression analysis. Probability values of p < 0.05 were considered to be significant.
RESULTS
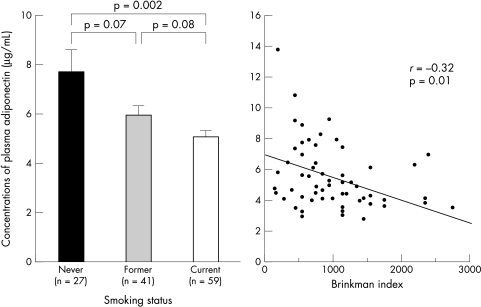
In this study group, never, former, and current smokers comprised 27 patients (21%), 41 patients (32%), and 59 patients (47%), respectively. Plasma concentrations of adiponectin decreased in a stepwise fashion for never, past, and current smokers (mean (SD) 7.7 (5.5), 5.9 (2.7), and 5.0 (2.2) μg/ml, p for trend = 0.003) as shown in fig 1. Compared with never smokers, plasma concentrations of adiponectin were significantly lower in current smokers (p = 0.002), and tended to be lower in former smokers (p = 0.07). Compared with former smokers, plasma concentrations of adiponectin also tended to be lower in current smokers (p = 0.08). Body mass index was not significantly different among these three groups (24.3 (2.6), 24.5 (3.1), and 23.9 (3.9) kg/m2, p = ns). HOMA-IR values were also not significantly different among these three groups (2.2 (1.6), 2.2 (1.8), and 2.2 (1.6), p = ns). In addition, plasma concentrations of adiponectin were negatively correlated with smoking index (Brinkman index: number of cigarettes smoked per day × number of years of the habit) in current smokers (r = 0.32, p = 0.01) as shown in fig 1.
Figure 1.
Smoking status and concentrations of plasma adiponectin Brinkman index: number of cigarettes smoked per day × number of years of the habit. Error bars = SEM.
DISCUSSION
This study demonstrated that plasma concentrations of adiponectin were associated with smoking status in patients with CAD regardless of their body mass index and HOMA-IR. One possible mechanism by which smoking could lead to decreased circulating adiponectin concentrations is via an increase in the activity of the sympathetic nervous system secondary to the effects of nicotine to activate post-ganglionic sympathetic nerves. Indeed, it was reported that adrenergic agonists or cyclic AMP analogues inhibit adiponectin gene expression.6 Another mechanism, which is indirect, might be the consumption of circulating adiponectin by means of binding to some molecules in injured vessels damaged by smoking and/or components of smoking. Adiponectin has been reported to have binding ability to collagens I, III, and V, which are abundant in the vascular intima, and to accumulate in the injured vascular walls.7 Therefore, decreased adiponectin caused by smoking may play an important role in the pathophysiology of coronary atherosclerosis.
REFERENCES
- 1.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 2002;13:51–9. [DOI] [PubMed] [Google Scholar]
- 2.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999:257;79–83. [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E. Adiponectin enhances insulin action by decreasing ectopic fat deposition. Pharmacogenomics J 2002:2:4–7. [DOI] [PubMed] [Google Scholar]
- 4.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002;8:731–7. [DOI] [PubMed] [Google Scholar]
- 5.Kilaru S, Frangos SG, Chen AH, et al. Nicotine: a review of its role in atherosclerosis. J Am Coll Surg 2001;193:538–46. [DOI] [PubMed] [Google Scholar]
- 6.Delporte ML, Funahashi T, Takahashi M, et al. Pre- and post-translational negative effect of beta-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem J 2002;367:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res 2000;32:47–50. [DOI] [PubMed] [Google Scholar]