As the human immunodeficiency virus (HIV) pandemic enters its third decade, the resultant acquired immunodeficiency syndrome (AIDS) is a global health crisis with approximately 36 million people affected worldwide.1,2 The disease is now the principal cause of death in young adults in many parts of the USA and Europe, and morbidity and mortality have risen sixfold in sub-Saharan Africa where modern health care is unavailable to many. Furthermore, over one million children worldwide are living with HIV/AIDS and 1600 babies are born with HIV infection every day.
Early therapeutic goals focused on prolongation of life by aggressive treatment of often fatal opportunistic infections, such as Pneumocystis carinii pneumonia. Descriptions of specific HIV related myocarditis and cardiomyopathy appeared during the 1980s and overall mortality fell with improved prophylactic regimens against opportunistic infection and clinical surveillance programmes. In the mid 1990s the advent of combination highly active antiretroviral therapy (HAART) made a major impact on the morbidity and mortality of HIV patients. Combination therapy reduces viral replication, delays disease progression, and prolongs survival, while limiting development of viral resistance. Survival to 40–50 years is no longer unusual and coronary artery disease, either de novo or as an iatrogenic consequence of newer treatment regimens, is emerging as an important problem.
HIV infection is characterised by an acquired, irreversible, profound immunosuppression that predisposes patients to multiple opportunistic infections, malignancies, and progressive dysfunction of multiple organ systems. HIV specifically infects and gradually depletes CD4+ lymphocytesw1 but may also affect other cell types, including monocytes/macrophages, endothelial cells, glial cells, intestinal epithelial cells, and possibly neurons. Studies have suggested that HIV may exhibit a cardiac tropism,w2 but the heart may also be affected by other opportunistic viruses, fungi, and protozoa. Cardiac disease associated with HIV may therefore be multifactorial, and can be caused by infectious or neoplastic complications or their treatments, any of the established causes of cardiac disease in other patient populations, or by HIV infection of the myocardium itself. Knowledge of the relative frequency of each form of heart disease in patients with HIV is constantly evolving and encapsulated in a series of review articles published over the past decade.2–7 w3–8 This article presents a review of our knowledge to date.
CARDIAC INVOLVEMENT IN HIV PATIENTS
Cardiac involvement in AIDS was first reported in 1983 in a postmortem description of a 24 year old woman of Haitian origin with multiple complications of AIDS, including Kaposi’s sarcoma involving the entire anterior cardiac wall without pericardial effusion.8 Subsequently, cardiac involvement in patients with HIV infection has been described in multiple necropsy, clinical, and echocardiographic series. Almost any agent that can cause disseminated infection in patients with AIDS may involve the myocardium, but clinical evidence of cardiac disease is usually overshadowed by manifestations in other organs, primarily the brain and lungs. Thus, the number of patients with AIDS and cardiac involvement at necropsy greatly exceeds the number with significant cardiac disease during life. Estimates of prevalence vary widely from 28–73%1 according to the screening methods selected. Although exact data are unavailable, conservative estimates derived from European and US series indicate cardiac morbidity and mortality in HIV patients of 6–10% and 1–9%, respectively.3,4 Important clinical syndromes involving the heart that have been described in patients with HIV infection are summarised in table 1.
Table 1.
Cardiac conditions associated with HIV infection
| Myocardial disease | ||
| Opportunistic infections | ||
| Bacterial | ||
| Mycobacterium tuberculosis, Mycobacterium avium-intracellulare | ||
| Fungal | ||
| Cryptococcus neoformans, Aspergillus fumigatus, Candida albicans, Histoplasma capsulatum, Coccidiodes immitus | ||
| Protozoan | ||
| Toxoplasma gondii, Pneumocystis carinii | ||
| Viral | ||
| Cytomegalovirus, herpes simplex, coxsackie B virus | ||
| Direct HIV infection | ||
| Lymphocytic myocarditis | ||
| Non-inflammatory myocardial necrosis | ||
| Microvascular spasm | ||
| Cathecholamine excess | ||
| Nutritional deficiency (selenium, thiamine) | ||
| Hypoxic injury | ||
| Drug toxicity | ||
| Anti-HIV drugs | ||
| Drugs used for opportunistic infections | ||
| Arteriopathy | ||
| Cardiomegaly and cardiomyopathy | ||
| Congestive cardiomyopathy | ||
| Right ventricular hypertrophy/dilatation with pulmonary hypertension: | ||
| pulmonary infection | ||
| pulmonary emboli | ||
| HIV plexogenic pulmonary arteriopathy | ||
| Neoplasia | ||
| Kaposi’s sarcoma | ||
| Lymphoma | ||
| Endocardial disease | ||
| Marantic endocarditis (non-bacterial thrombotic endocarditis) | ||
| Healed infective endocarditis | ||
| Infective endocarditis | ||
| Bacterial | ||
| Fungal | ||
| Pericardial disease | ||
| Infectious | ||
| Bacterial | ||
| Tuberculosis (M tuberculosis, M avium-intracellulare), Nocardia asteroides, Klebsiella pneumoniae, Staphylococcus aureus | ||
| Viral | ||
| Herpes simplex, HIV, coxsackie, cytomegalovirus | ||
| Fungal | ||
| Histoplasma, cryptococcus | ||
| Uraemia | ||
| Neoplastic | ||
| Kaposi’s sarcoma | ||
| Lymphoma | ||
| Fibrinous pericarditis | ||
| Idiopathic | ||
| Vascular lesions | ||
| Fibrocalcific degenerative arteriopathy | ||
| Vasculitis, perivasculitis | ||
| Aneurysms | ||
| Atherosclerotic lesions | ||
The infecting organisms listed here are not exhaustive and are examples only.
HIV, human immunodeficiency virus.
Adapted with permission from Arshad et al.3
MYOCARDIAL DISEASE
The first case of rapidly fatal, dilated cardiomyopathy in a patient with AIDS was described in 1986.w9 Among patients with dilated cardiomyopathy, HIV is the underlying cause in 4%6; prognosis is poor and patients with HIV related cardiomyopathy have a mortality hazard ratio of 4.0 in comparison with controls with idiopathic dilated cardiomyopathy.w10 Dilated cardiomyopathy affects 10–20% of those with HIV infection and accounts for approximately a third of HIV related deaths.7 Median survival is 101 days in patients with left ventricular dysfunction compared with 472 days in HIV patients with a normal echocardiogram at the same stage of infection.w11 Similarly, a longitudinal, prospective study of HIV infected infants and children found that left ventricular dysfunction was a significant predictor of overall mortality, even after adjustment for age, height, CD4 cell count, and progressive neurological disease.9
Mechanisms
Dilated cardiomyopathy occurs late in the course of HIV infection and is usually associated with a significantly reduced CD4 count.w11 Pathological examination shows endocardial fibrosis and mural thrombus, particularly at the apex, and histological evidence of myocyte hypertrophy and degeneration with increased interstitial and endocardial fibrillar collagen, often associated with evidence of previous myocarditis (fig 1). The pathogenesis remains uncertain: speculated causes include direct infection of the heart by HIV itself, toxic effects of antiretroviral therapy, effects of circulating or systemic toxins, infection of the heart by opportunistic pathogens, toxicity of alcohol, illicit or self prescribed substances, and nutritional disorders. Indeed, several of these factors may operate in an individual patient.
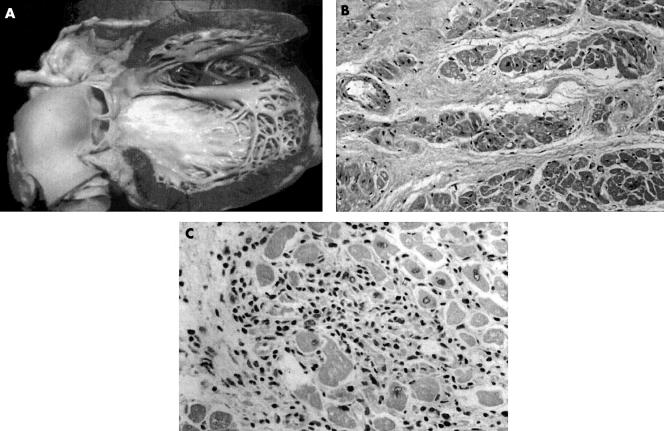
Figure 1.
HIV related cardiomyopathy. (A) The heart is enlarged, principally from ventricular dilatation, and there is mild hypertrophy with diffuse endocardial fibrous thickening. Histological examination (B) may reveal myocyte hypertrophy with increased interstitial collagen or (C) evidence of myocarditis with lymphocytic infiltrate and myocyte necrosis. Reproduced with permission from D’Amati et al.5 Copyright 2001 New York Academy of Sciences, USA.
Direct correlation between histological cardiac abnormalities and clinical or functional heart muscle disease has not been established, making it difficult to determine whether cardiomyopathy is related directly to the presence of the virus or triggering of overt myocarditis. In one prospective study of 952 asymptomatic HIV positive patients, an echocardiographic diagnosis of dilated cardiomyopathy was made in 76 (8%) patients with a mean annual incidence of 15.9/1000 patients. All patients with echocardiographic abnormalities underwent myocardial biopsy: myocarditis was present in 63 (83%) of the patients with dilated cardiomyopathy, and 36 (57%) of these had a positive hybridisation signal for HIV. Co-infection with coxsackie virus, cytomegalovirus, and Epstein-Barr virus was also noted in many cases.10 HIV virions may infect cardiac myocytes resulting in injury as a result of direct toxicity or activation of multifunctional cytokines (for example, endothelin-1, tumour necrosis factor α, interleukin-6).w10 The mode of entry of HIV into myocytes remains unclear since they are CD4 receptor negative. Further putative mechanisms of tissue damage include postviral autoimmunity and immune system dysregulation,w12 adverse effects of viral proteins (including apoptosis), interference with β adrenergic stimulation, and transcriptional activation of cellular genes.w13
HIV infection may persist in reservoir cells within the myocardium and cerebral cortex despite antiretroviral therapy and be associated with chronic release of cytotoxic cytokines.w14 Thus, patients with encephalopathy have a higher likelihood of death from congestive heart failure than non-encephalopathic controls (hazard ratio 3.4). Malnutrition and wasting are also important predictors of cardiac morbidity and mortality in HIV infection. There is a relation between trace element deficiency and cardiomyopathy, and the cardiac virulence of coxsackie virus is enhanced by selenium deficiency. Indeed, selenium supplementation has been shown to improve cardiac dysfunction in AIDS patients.w15
Investigation
Echocardiography is helpful in the diagnosis of dilated cardiomyopathy and may also detect diastolic dysfunction in early disease.w16 Computed tomography or magnetic resonance imaging may help to clarify the aetiology, especially in cases secondary to neoplastic infiltration. The need for routine myocardial biopsy is controversial and associated risks are significant—sensitivity is low, especially in patchy lesions, and beyond research protocols its use is limited to patients with extensive cardiac damage with no identifiable cause.
Treatment
Treatment for HIV related cardiomyopathy is generally similar to that for non-ischaemic cardiomyopathy. Angiotensin converting enzyme inhibitors and β blockers are recommended but may be poorly tolerated because of low systemic vascular resistance from diarrhoeal disease, infection or dehydration. Patients with myocarditis have enhanced sensitivity to digoxin and anticoagulation presents risks to patients with cerebral vasculopathy and possible aneurysm formation. The use of immunosuppressive regimens is controversial and no convincing benefits have been reported other than with intravenous immunoglobulin,11 whose efficacy may reflect inhibition of cardiac autoantibodies by competition with Fc receptors or dampened effects of cytokines and cellular growth factors.w17
ENDOCARDIAL DISEASE
Three forms of endocarditis have been reported in HIV infected patients: marantic (non-bacterial thrombotic), bacterial, and fungal.
Marantic endocarditis
Marantic endocarditis can involve all four cardiac valves though left sided lesions are more common. Vegetations are friable, consisting of platelets within a fibrin mesh with a few inflammatory cells, and systemic embolism is common.w18 The condition, which is usually associated with hypercoagulable states in systemic lupus erythematosus, disseminated intravascular coagulation, and malignancy, is difficult to diagnose ante-mortem. Identification was frequent in early postmortem studies of patients with HIV infection, but the condition is now less commonly encountered, suggesting that its prevalence was overestimated in the past.
Bacterial endocarditis
Bacterial endocarditis in HIV infection is infrequent, appearing almost exclusively in intravenous drug users where prevalence varies from 6.3–34%.2 In North America, where most HIV affected patients are homosexuals, endocarditis is uncommon. Intravenous drug users have frequent bacteraemias owing to the introduction of skin pathogens and talcum powder by unsterile intravenous injection. Vegetations form on the tricuspid and pulmonary valve with resultant pulmonary embolism and septic pulmonary infarction (fig 2). Patients typically present with fever, sweats, weight loss, and co-existing pneumonia and/or meningitis. Infection affecting the left heart with systemic embolism is less common. Overall incidence of endocarditis in this group is falling, an unexpected benefit of needle exchange and health education schemes,12 and overdose is a more common cause of death.w19 Staphylococcus aureus is the most common organism (> 75%) followed by Streptococcus pneumoniae and Haemophilus influenzae, although these patients are also at increased risk of Salmonella infection.w20 Methicillin resistant staphylococci have been reported, and the HACEK group of organisms (Haemophilus aphrophilus, Haemophilus paraphrophilus, Haemophilus influenzae, Haemophilus parainfluenzae, Actino-bacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella species) should be considered in cases which are resistant to culture. Austrian’s syndrome, characterised by acute streptococcal endocarditis, pneumococcal pneumonia, and meningitis, originally described in alcoholics, is also more common in AIDS patients.w21
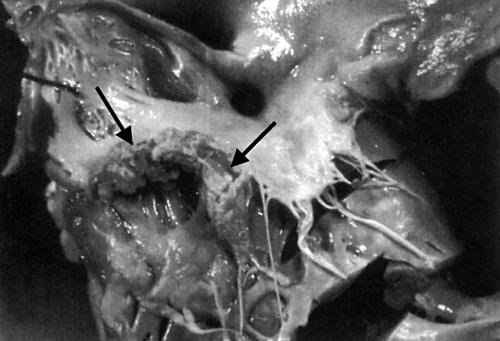
Figure 2.
Infective endocarditis of the tricuspid valve in an HIV positive drug user with a large polypoid thrombotic lesion attached to the posterior leaflet (arrow). Reproduced with permission from D’Amati et al.5 Copyright 2001 New York Academy of Sciences, USA.
Fungal endocarditis
Fungal endocarditis is usually the result of systemic fungaemia. Aspergillus endocarditis has been reported in association with pulmonary aspergillosis, and cryptococcal and candidal endocarditis may complicate primary infection in the oropharynx and oesophagus, particularly in patients nursed on intensive care units.
Excepting the late stages of AIDS, HIV infection appears to have little effect on susceptibility to or mortality from endocarditis, and aggressive treatment with conventional antibiotic regimens and surgery when required are appropriate. Medical treatment is successful in > 70% of cases and surgery also has good outcome, provided that intravenous drug abuse does not resume in the postoperative period.w22
PERICARDIAL DISEASE
Pericardial disease is a frequent cardiovascular manifestation of HIV infection often associated with shortened survival, independent of CD4 count and albumin values.6,13 The prevalence of pericardial disease at echocardiography ranges from 10–59%3 and was 21% in the largest series of 1139 patients.13 In Africa and many US urban settings, pericardial effusion associated with HIV is now the most frequent cause of pericardial disease.w23 w24 The clinical spectrum includes effusions with and without cardiac tamponade, constrictive pericarditis, and neoplastic infiltration by lymphoma and Kaposi’s sarcoma. There is no apparent correlation between clinical stage of HIV infection and severity of pericardial involvement.w25
Pericarditis is usually non-specific in origin and can occur with or without effusion. Initial symptoms and signs may be subtle but include chest pain and signs of tamponade and hypotension. Low pressure tamponade may be encountered in patients with AIDS who are severely dehydrated and cachectic with low resting right ventricular filling pressures. The cause of pericardial effusion is frequently difficult to determine though it may be associated with Kaposi’s sarcoma, lymphoma, and bacterial (Mycobacterium species, Nocardia species, Staphylococcus aureus), viral (herpes simplex, cytomegalovirus), and fungal (Cryptococcus neoformans) infection. In one typical series of 66 HIV patients with cardiac tamponade, 26% were caused by tuberculosis, 17% were purulent, 8% were caused by atypical mycobacteria, and 10% by Kaposi’s sarcoma and lymphoma.w26 HIV itself has been isolated from macrophages within the pericardial fluid of one patient, though its role remains uncertain.w27 Culture of pericardial fluid is often unrevealing and effusion may be part of a generalised serous effusive process also involving pleural and peritoneal surfaces, possibly related to enhanced expression of cytokines (for example, tumour necrosis factor α) in the later stages of HIV disease.6
Small asymptomatic effusions do not require diagnostic evaluation and spontaneously resolve in up to 42% of patients.6 Tuberculosis in AIDS is often atypical with a high prevalence of extrapulmonary manifestations, and in areas such as Africa where the incidence of tuberculous infection is high, patients with pericardial effusion often receive empirical antituberculous chemotherapy. Pericardiocentesis is currently recommended for patients with large or poorly tolerated effusions, diagnostic assessment in the presence of systemic illness, or cardiac tamponade. The effects of HAART on pericardial effusion are unknown.
MALIGNANT DISEASE
Two types of malignancy affect the heart in HIV patients: Kaposi’s sarcoma, and malignant lymphoma, of which the former is more common.w28
Kaposi’s sarcoma
Kaposi’s sarcoma is a low grade neoplasm arising from mesenchymal or endothelial cells and occurs in approximately 30% of AIDS patients, mostly male homosexuals. Cardiac involvement usually reflects a widely disseminated process. Visceral and parietal pericardial lesions are most common though involvement of the myocardium, coronary arterial adventitia, great vessels, epicardium, and epicardial fat has also been described.w18 Nodular coalescent dark red or violaceous plaques are characteristic (fig 3A) and histological examination reveals nodular lesions formed by spindle cells surrounding slit-like capillary vessels. Cardiac Kaposi’s sarcoma is usually occult and rarely diagnosed during life. Pericardial involvement may rarely manifest as tamponade or constriction and underlying myocardial function is rarely affected.
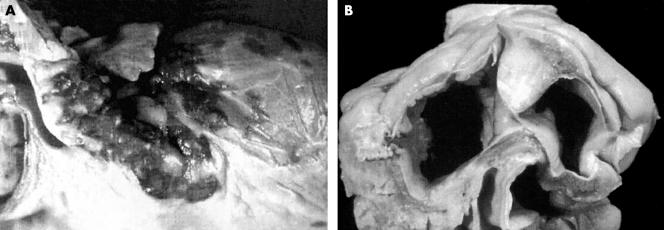
Figure 3.
(A) Cardiac Kaposi’s sarcoma with typical red purple coalescent epicardial plaques. Histological examination reveals spindle cells surrounding slit-like capillary vessels. (B) HIV related large cell B immunoblastic lymphoma involving the interatrial septum. Histological examination demonstrates lymphomatous myocardial infiltration associated with diffuse myocardial damage. Reproduced with permission from D’Amati et al.5 Copyright 2001 New York Academy of Sciences, USA.
Malignant lymphoma
The frequency of non-Hodgkin’s lymphoma in patients with AIDS is increasing and estimated to be 25–60 times greater than expected in the general population. Lymphomas are observed in 5–10% of patients infected with HIV and constitute the first manifestation of AIDS in 3–4% of new cases.14 Most non-Hodgkin’s lymphomas affecting the heart in HIV infection are high grade, with Burkitt-like cells, reticular cell sarcomas, or large cell immunoblastic sarcomas. The majority originate from B cells and display monoclonal immunoglobulin staining. Pathological examination demonstrates pallor of the heart secondary to diffuse lymphomatous infiltration or patchy involvement of the epicardium, myocardium, and endocardium in the form of focal circumscribed nodules, most frequently affecting the right atrium (fig 3B). Histological sections contain infiltrates of plasmacytoid lymphocytic cells, resembling immunoblastic non-Hodgkin’s lymphoma, and evidence of intense mitotic activity. Extranodal sites of lymphoma (typically the central nervous system, gastrointestinal tract, and bone marrow) are common and metastatic cardiac involvement may reflect this dissemination. Secondary cardiac involvement may also arise by direct extension of a mediastinal mass through the pericardium. Primary cardiac lymphoma is extremely rare but has been reported in patients with HIV.w29
Clinical manifestations of cardiac lymphoma include cardiomegaly, pericardial effusion, congestive heart failure, arrhythmias or progressive heart block. Sudden death is rare and most patients have no evidence of cardiac dysfunction. Outcome is usually poor and the optimal approach to treatment has yet to be determined, though clinical remission has been obtained with combination chemotherapy.14
VASCULAR DISEASE
Arterial lesions associated with HIV infection have been reported in children and adults and include arteriopathy with and without aneurysm formation, fibrocalcific lesions, and endothelial proliferation in association with Kaposi’s sarcoma. Inflammatory lesions (vasculitis or perivasculitis) may affect vessels in the brain and myocardium whereas fibrocalcific lesions, consisting of intimal fibrosis with medial calcification, aneurysm formation, and variable luminal narrowing are usually more widespread.w30 Premature atherosclerosis has also been observed in young adults in the absence of major risk factors or treatment with protease inhibitors. The pathogenesis is unclear but HIV itself may be implicated.w31 The observation of vascular disease in children and young adults suggests that arteriopathy will be seen with increasing frequency and severity as prolonged survival is achieved in HIV infection.
DRUGS AND CARDIOTOXICITY
Until recently, the prognosis of AIDS was so poor that concerns about long term effects of drug treatment were minor. The advent of potent antiretroviral drugs has had an impressive effect on mortality, disease progression, and incidence of HIV related disorders. Indeed, HIV infection should be considered a chronic condition in an increasing proportion of patients, and issues relating to long term drug treatment are of increasing relevance.15 A most important field of research is the possible increase of cardiovascular risk in patients treated with protease inhibitors.
Antiretroviral therapy
There are three classes of drugs presently used for people with HIV infection (table 2): nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors. The current standard of treatment is a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor combined with two nucleoside reverse transcriptase inhibitors, although modifications of this regimen are in constant evolution.w32
Table 2.
Antiretroviral drugs
| Nucleoside transcriptase inhibitors | Non-nucleoside transcriptase inhibitors | Protease inhibitors |
| Zidovudine | Nevirapine | Saquinavir |
| Didanosine | Delavirdine | Ritonavir |
| Zalcitabine | Efavirenz | Indinavir |
| Stavudine | Nelfinavir | |
| Lamivudine | Amprenavir | |
| Abacavir | Lopinavir |
Cardiomyopathy
Zidovudine was the first widely available antiretroviral drug and is still used in pregnant mothers who are HIV positive (and their newborns) and in combination regimens. Drug induced dilated cardiomyopathy secondary to mitochondrial toxicity has been associated with its use in adults and children.
Dyslipidaemia and cardiovascular risk
Hyperlipidaemia, hyperglycaemia, hyperinsulinaemia, and lipodystrophy are frequent adverse effects of potent antiretroviral combination therapy, particularly involving protease inhibitors.16 Hyperlipidaemia affects approximately 50% of patients using protease inhibitors, with average increases in total cholesterol and triglyceride concentrations of 28% and 96%, respectively.w33 The degree of increase seems to be proportional to the duration of treatment and type of drug.
Recent reports of myocardial infarction in young patients receiving protease inhibitors have focused interest on the association between HIV infection, antiretroviral therapy, and coronary artery disease. For example, in a small French study, patients treated with protease inhibitors had an almost threefold increase in risk of myocardial infarction compared with untreated controls, suggesting that rapidly forming drug induced plaques are unstable and prone to rupture.w34 Larger studies addressing the underlying abnormalities of endothelial function and anticoagulation and broader epidemiological issues are underway.
Preliminary guidelines for the evaluation and management of dyslipidaemia in HIV positive patients receiving HAART have recently been published.17 Key points are routine screening, lifestyle advice (including smoking cessation), comprehensive lipid analysis before commencing antiretroviral therapy, and treatment of selected cases. Statins which are independent of the cytochrome P450 system (for example, pravastatin, atorvastatin) are recommended to avoid interaction with protease inhibitors, and use of fibrates and gemfibrozil has been described in patients with isolated hypertriglyceridaemia.
In some patients, with high atherosclerotic co-morbidity or risk, a high CD4+ cell count, and low viral load, the risk:benefit ratio of treatment with HAART may be questionable.
Drugs used for HIV related disorders
Drugs used for HIV related diseases may have adverse cardiac effects including cardiomyopathy, QTc prolongation with increased risk of arrhythmia, and acceleration of coronary disease (table 3). Arrhythmias may be precipitated in QTc prolongation by concomitant use of drugs that share the CY3PA metabolic pathway (including protease inhibitors) and clinicians should be aware of this potentially dangerous interaction.
Table 3.
Drugs with potential cardiotoxicity used in HIV
| Drug | Use in HIV | Cardiotoxicity | |
| Nucleoside transcriptase inhibitors | HIV disease | Cardiomyopathy | |
| Non-nucleoside transcriptase inhibitors | HIV disease | Increased coronary vascular risk | |
| Protease inhibitors | HIV disease | Increased coronary vascular risk | |
| Anti-infective drugs | Opportunistic infections | ||
| Erythromycin | Prolonged QT interval | ||
| Trimethoprim/sulfamethoxazole | Prolonged QT interval | ||
| Clarithromycin | Prolonged QT interval | ||
| Pentamidine | Prolonged QT interval | ||
| Ventricular arrhythmias | |||
| Orthostatic hypotension | |||
| Pyrimethamine | Prolonged QT interval | ||
| Fluoroquinolones | Prolonged QT interval | ||
| Amphotericin B | Prolonged QT interval | ||
| Hypokalaemia | |||
| Bradycardia | |||
| Hypertension | |||
| Azole antifungals | Prolonged QT interval | ||
| Ganciclovir | Ventricular arrhythmias | ||
| Foscarnet | Cardiomyopathy | ||
| Psychotropic drugs | Psychotic disorders, depression | ||
| Tricyclic antidepressants | Prolonged QT interval | ||
| Phenothiazines | Prolonged QT interval | ||
| Antihistamines | Allergic reactions | ||
| Astemizole | Prolonged QT interval | ||
| Terfenadine | Prolonged QT interval | ||
| Chemotherapy regimens | HIV related neoplasms | ||
| Anthracyclines | Cardiomyopathy (acute/chronic) | ||
| Ventricular arrhythmias | |||
| Heart block | |||
| Vincristine | Hypertension | ||
| Coronary heart disease | |||
| Interferon α | Kaposi’s sarcoma, | Cardiomyopathy | |
| Hepatitis C infection | Coronary heart disease | ||
| Interleukin-2 | HIV disease | Cardiomyopathy | |
| Coronary heart disease | |||
| Erythropoeitin α | Chronic anaemia | Hypertension | |
| Cocaine | Recreational | Hypertension | |
| Coronary heart disease (dissection) | |||
| Cardiomyopathy | |||
| Myocarditis | |||
| Anabolic steroids | Weight gain/recreational | Coronary heart disease | |
MISCELLANEOUS
Paediatric disease
As the number of children living with HIV increases so will the number that suffer with cardiovascular complications. Cardiac mortality increases in frequency with increasing age (fig 4). The vast majority of HIV positive children acquired the virus soon or after birth. The rate of vertical transmission has substantially decreased in recent years owing to administration of antiretroviral therapy during pregnancy and delivery. Fetal echocardiography has demonstrated that fetuses of HIV positive mothers have abnormal cardiovascular structure and function, including increased right and left ventricular wall thickness. The incidence of congenital cardiovascular malformations does not differ from matched HIV negative controls.18 w35 Frequently encountered cardiovascular manifestations of HIV infection in children include increased left ventricular mass with systolic dysfunction, myocarditis (usually related to adenovirus and cytomegalovirus), vascular disease, aortic root dilatation, pericardial effusions, tumours, and minor ECG abnormalities.18 Encephalopathy, wasting, decreased CD4 count, Epstein-Barr virus infection, and a prior history of a serious cardiac event all predict cardiac complications of HIV infection in children.w36
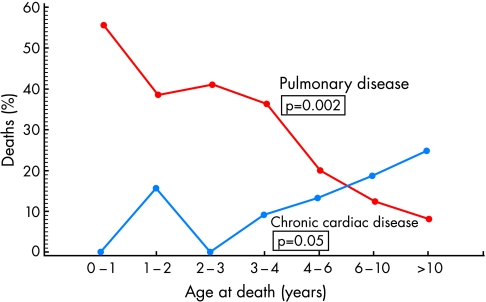
Figure 4.
Linear age trends for the underlying cause of 93 HIV related deaths in children. Reproduced with permission from Langston C et al. Human immunodeficiency virus-related mortality in infants and children: data from the pediatric pulmonary and cardiovascular complications of vertically transmitted HIV (P2C2) study. Copyright Pediatrics 2001;107:328–38.
HIV and cardiovascular medicine: key points.
Approximately 36 million people worldwide are infected with HIV, including over one million children
Significant cardiac disease affects 10–20% of patients infected with HIV and is the cause of death in approximately 5%
HIV related cardiomyopathy has a poor prognosis, especially in patients with a low CD4 count or coexistent encephalopathy; pathogenesis is multifactorial and treatment options are limited
Other frequent cardiac manifestations of HIV infection include opportunistic infection, infective endocarditis (especially affecting intravenous drug users), pericardial effusion, neoplasm (Kaposi’s sarcoma/lymphoma), and pulmonary hypertension
Premature vascular disease is an emerging problem, especially in patients receiving combination antiretroviral therapy
Cardiac surgery is well tolerated by patients with HIV/AIDS. The risks of transmission to the surgeon are low
Pulmonary hypertension and right ventricular dysfunction
Primary pulmonary hypertension has been reported in HIV infected patients without evidence of thromboembolic disease, intravenous drug use, right sided endocarditis or pulmonary infection.w37 Development and progression bear no relation to the stage of underlying HIV disease. It affects about 0.5% of hospitalised AIDS patients and is a cause of severe cardiac impairment with associated cor pulmonale and death.6 Histological examination most frequently demonstrates plexogenic pulmonary arteriopathy. The pathogenesis is multifactorial and poorly understood: HIV may cause endothelial damage and vasoconstriction through release of endothelin-1, interleukin-6, and tumour necrosis factor α. HIV may also be identified in alveolar macrophages which release tumour necrosis factor α, oxide anions, and proteolytic enzymes in response to infection. Therapeutic effects of oxygen, steroids, calcium channel blockers, epoprostenol, and nitric oxide have all been proposed though efficacy has not been confirmed in controlled clinical trials.19 Effects of HAART on pulmonary artery endothelial cells are unknown.
Autonomic dysfunction
HIV infection may be associated with abnormalities of the autonomic nervous system, particularly in advanced disease. Cardiovascular autonomic reflexes may be profoundly affected causing postural hypotension, syncope, and cardiorespiratory arrest during invasive procedures. Prevalence varies from 5–77%, according to definition.w38 The cause is unknown although HIV is neurotropic and has been isolated from peripheral nerve tissue. Underlying myocardial disease is not implicated.w39
CARDIAC SURGERY
As long term survival improves, the number of potential patients with degenerative and acquired coronary artery and valvar heart disease coming forward for cardiac surgery rises. As experience in this difficult area increases, initial fears that cardiopulmonary bypass might accelerate progression of HIV infection to AIDS and its complications have not been borne out.w40 HIV infected patients and those with established AIDS have shown considerable tolerance to major cardiac and pulmonary surgery. Survival and complication rates do not differ significantly from those reported in HIV negative patients, although recurrent endocarditis remains a problem in intravenous drug addicts unable to refrain from continued high risk behaviour.20
Concerns that surgeons would be exposed to significant risk of acquiring HIV infection have also been unfounded. With drug induced preoperative reduction of viral load, use of appropriate precautions and chemoprophylaxis in the event of needlestick injury, no definite transmission to a surgeon during cardiac surgery has been reported. Paradoxically, surveys indicate widespread fear of HIV infection and poor knowledge of and compliance with guidelines for universal precautions, exposing surgeons to the higher risks of hepatitis B and C which are more easily transmitted than AIDS and may be fatal.20 Similar ignorance has been reported in the attitudes of hospital doctors towards mouth-to-mouth resuscitation.w41
CONCLUSION
With current advances in HIV/AIDS management and increased survival, cardiac manifestations of HIV disease will become more important and encountered more frequently. HAART is only available to a minority of HIV infected individuals worldwide and studies from the pre-HAART period still apply. Since cardiac complications are often clinically inapparent, periodic screening of HIV positive patients by means of a three monthly ECG and six monthly echocardiogram is recommended, especially in those with low CD4 counts or receiving treatment with zidovudine. Risk factors for vascular disease should be monitored in patients receiving HAART.
The heart may be a marker of the HIV infected patient’s overall health, and a decline in cardiac function should trigger more comprehensive evaluation. As the role of infection and inflammation in many other cardiovascular diseases is now recognised, identification of the molecular mechanisms of HIV related heart disease may have broader implications for a wide range of patients.
Supplementary Material
REFERENCES
- 1.Lewis W. Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis 2000;43:151–70. ▸ A detailed review of the pathogenesis, pathophysiology, and epidemiology of HIV related cardiomyopathy with summaries of recent basic scientific advances and proposed in vivo models. Contains 226 references. [DOI] [PubMed] [Google Scholar]
- 2.Barbaro G, Fisher SD, Pellicelli AM, et al. The expanding role of the cardiologist in the care of HIV infected patients. Heart 2001;86:365–7. ▸ A concise review article summarising the everyday cardiac complications of HIV infection likely to present to the busy practising cardiologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arshad A, Bansal A, Patel RC. Cardiac complications of human immunodeficiency virus infection: diagnostic and therapeutic considerations. Heart Disease 2000;2;133–45. ▸ A more detailed comprehensive review outlining the cardiac complications of HIV infection described to date. Contains 162 references. [PubMed] [Google Scholar]
- 4.Yunis NA, Stone VE. Cardiac manifestations of HIV/AIDS: a review of disease spectrum and clinical management. J Acquir Immune Defic Syndr Hum Retrovirol 1998;18:145–54. [DOI] [PubMed] [Google Scholar]
- 5.D’Amati G, Di Gioia CR, Gallo P. Pathological findings of HIV-associated cardiovascular disease. Ann N Y Acad Sci 2001;946;23–45. ▸ A superbly illustrated review focusing on HIV associated cardiovascular pathology, correlating the morphologic findings to clinical syndromes of HIV and AIDS. [DOI] [PubMed] [Google Scholar]
- 6.Rerkpattanapipat P, Wongpraparut N, Jacobs LE, et al. Cardiac manifestations of acquired immunodeficiency syndrome. Arch Intern Med 2000;160:602–8. [DOI] [PubMed] [Google Scholar]
- 7.Milei J, Grana D, Fernández Alonso G, et al. Cardiac involvement in acquired immunodeficiency syndrome – a review to push action. Clin Cardiol 1998;21:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autran BR, Gorin I, Leibowitch M, et al. AIDS in a Haitian woman with cardiac Kaposi’s sarcoma and Whipple’s disease. Lancet 1983;i:767–8. ▸ The first clinical description of cardiovascular disease complicating HIV infection. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Easley KA, Orav EJ, et al. Cardiac dysfunction and mortality in HIV-infected children. The prospective P2C2 HIV multicenter study. Circulation 2000;102;1542–8. ▸ A landmark paper describing the outcome of the first major epidemiological study of cardiovascular problems associated with HIV infection in a cohort of vertically infected children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro G, Di Lorenzo G, Grisorio B, et al. Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV-positive patients. N Engl J Med 1998;339;1093–9. ▸ A prospective long term clinical and echocardiographic follow up study of 952 asymptomatic HIV positive patients reporting the incidence of dilated cardiomyopathy and demonstrating a direct pathological role of HIV. [DOI] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Orav EJ, Sanders SP, et al. Immunoglobulins and left ventricular structure and function in pediatric HIV infection. Circulation 1995;92:2220–5. [DOI] [PubMed] [Google Scholar]
- 12.Currie PF, Sutherland GR, Jacob AJ, et al. A review of endocarditis in acquired immunodeficiency syndrome and human immunodeficiency syndrome. Eur Heart J 1995;16(suppl B):15–18. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Eisenberg MJ, Kee LL, et al. Pericardial effusion in AIDS: incidence and survival. Circulation 1995;92:3229–34. [DOI] [PubMed] [Google Scholar]
- 14.Duong M, Dubois C, Buisson M, et al. Non-Hodgkin’s lymphoma of the heart in patients infected with human immunodeficiency virus. Clin Cardiol 1997;20:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantoni M, Autore C, Del Borgo C. Drugs and cardiotoxicity in HIV and AIDS. Ann N Y Acad Sci 2001;946;179–99. ▸ A comprehensive review of cardiovascular problems associated with drug therapy in HIV infection with special emphasis on premature vascular disease related to highly active antiretroviral therapy. Contains 142 references. [DOI] [PubMed] [Google Scholar]
- 16.Périard D, Telenti A, Sudre P, et al. Atherogenic dyslipidaemia in HIV-infected individuals treated with protease inhibitors. Circulation 1999;100:700–5. [DOI] [PubMed] [Google Scholar]
- 17.Dubé MP, Sprecher D, Henry WK, et al. Preliminary guidelines for the evaluation and management of dyslipidaemia in adults infected with human immunodeficiency virus and receiving antiretroviral therapy: recommendations of the adult AIDS clinical trial group cardiovascular disease focus group. Clin Infect Dis 2000;31;1216–24. ▸ Summary guidelines for the screening and treatment of vascular risk factors in patients scheduled for treatment with highly active antiretroviral therapy. [DOI] [PubMed] [Google Scholar]
- 18.Keesler MJ, Fisher SD, Lipshultz SE. Cardiac manifestations of HIV infection in infants and children. Ann N Y Acad Sci 2001;946:169–78. [DOI] [PubMed] [Google Scholar]
- 19.Mehta NJ, Khan IA, Mehta RN, et al. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 2000;118:1133–41. [DOI] [PubMed] [Google Scholar]
- 20.Frater RW. Cardiac surgery and the human immunodeficiency virus. Semin Thorac Cardiovasc Surg 2000;12;145–7. ▸ A discussion paper outlining current views on cardiac surgery in patients with HIV infection. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.